
Background: Measurable residual disease (MRD) can identify patients (pts) with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) in complete hematological remission (CR) at high risk of relapse even after allogeneic hematopoietic stem cell transplantation (HSCT). We have recently shown in 53 pts treated within the first cohort of the RELAZA2 trial that pre-emptive therapy with azacitidine (AZA) at the time of MRD-positivity (MRDpos) can successfully prevent imminent hematological relapse (Platzbecker et al. Lancet Oncol. 2018). We now report on the results of the second cohort of 41 pts undergoing MRD-guided treatment in the RELAZA2 trial (ClinicalTrials.gov NCT01462578) by the Study Alliance Leukemia (SAL).
Methods: Between 2015 and 2018, 166 MDS/AML pts were screened and centrally monitored for MRD in bone marrow or peripheral blood at monthly intervals for a period of 2 years prospectively in 9 centers in Germany. Of these 166, 41 pts with either advanced MDS (n=6) or AML (n=35) in CR after either conventional chemotherapy only (n=13) or consecutive allogeneic HSCT (n=28) developed MRD above a threshold defining imminent hematological relapse. Still being in morphological CR, these pts pre-emptively received 6 cycles of AZA (75mg/m2, s.c. days 1-7), which was followed by a risk-adapted AZA-maintenance therapy based on MRD-response for up to 18 additional months. Pts developing a hematological relapse went off study. MRD was detected by either the quantification of NPM1 mutation level (n=19), leukemia-specific fusion genes DEK-NUP214 (n=1) or RUNX1/RUNX1T1 (n=2) or a sensitive donor chimerism analysis of sorted CD34(+)/CD117(+) peripheral blood cells (n=28) in pts undergoing allogeneic HSCT. Here, we report the analysis of the primary endpoint of the 41 pts in the second cohort as well as the data for the entire 94 pts who entered the treatment phase of the RELAZA-2 study.
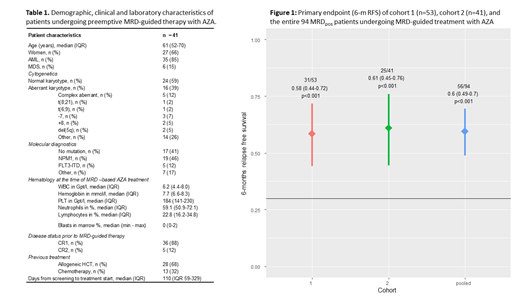
Results: At a median of 110 days (range 28-476) after start of screening, 41 (25%) out of 166 prospectively screened pts became MRDpos as defined by either a decrease of CD34(+)/CD117(+) donor chimerism to <80% (n=23) or a detectable mutation level >1% (NPM1 n=18) while being still in hematological CR. All of these MRDpos pts started AZA-based pre-emptive treatment to prevent imminent hematological relapse. Six months after start of MRD-guided therapy, 25 out of 41 pts were still in CR (61%, 95%-CI 45-76%, p<0.001, one-sided binomial test for H0: pexp≤0.3) while a total of 15 pts (37%) developed hematologic relapse after median of 3 AZA cycles. In fact, 19 pts (46%) responded with either a decline of MRD below a predefined threshold (increasing CD34(+) donor chimerism to ≥80% or mutation level <1%), while a stabilization in the absence of relapse was achieved in 6 pts (15%). Overall response rate was not statistically different between pts with (57%) or without (69%) antecedent allogeneic HSCT (p=0.5). After 6 months of initiation of MRD-guided treatment, 21 pts (51%) continued to receive a median of 6 (range 1-15) subsequent AZA cycles. Eventually, hematologic relapse occurred in 6 of those pts (29%), but was delayed until a median of 320 days (range 219-375 days) after initial MRD detection. With a median follow-up of 9 months after start of MRD-guided pre-emptive treatment the 12-months overall and progression free survival rate is 94% and 44%, respectively. When combining results for the primary endpoint with the first cohort, the 6 months relapse free survival for all 94 pts was 60% (56/94 pts.; 49-70%; p<.001 one-sided binomial test for H0: pexp≤0.3; Fig. 1).
Conclusion: These multicenter prospective data provide further strong evidence that continuous MRD monitoring is feasible and can identify MDS/AML pts at high risk of hematological relapse. Pre-emptive MRD-guided therapy with AZA is an effective treatment to prevent or at least substantially delay hematologic relapse in these pts.
Platzbecker:Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. Wolf:Celgene: Honoraria, Research Funding; Abbvie: Honoraria. Krämer:Daiichi-Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Research Funding. Bug:Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; Jazz Pharmaceuticals: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants; Hexal: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Sanofi: Other: travel grants; Celgene Neovii: Other: travel grant. Götze:AbbVie: Membership on an entity's Board of Directors or advisory committees. Stelljes:Novartis: Honoraria; Amgen: Honoraria; Jazz Pharmaceuticals: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; MDS: Consultancy. Subklewe:AMGEN: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Miltenyi: Research Funding; Oxford Biotherapeutics: Research Funding; Janssen: Consultancy; Roche: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Morphosys: Research Funding. Hänel:Celgene: Other: advisory board; Novartis: Honoraria; Takeda: Other: advisory board; Amgen: Honoraria; Roche: Honoraria. Dührsen:Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Alexion: Honoraria; Takeda: Consultancy, Honoraria; Teva: Honoraria; Celgene: Research Funding; Roche: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Janssen: Honoraria; Amgen: Consultancy, Honoraria, Research Funding; CPT: Consultancy, Honoraria. Müller-Tidow:MSD: Membership on an entity's Board of Directors or advisory committees. Thiede:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; AgenDix GmbH: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal