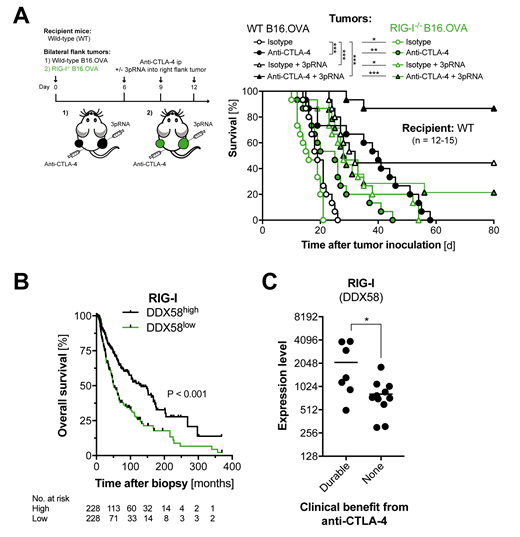
Achieving durable clinical responses to immune checkpoint inhibitors still remains a challenge. Here we demonstrate in preclinical models that immunotherapy with anti-CTLA-4 and its combination with anti-PD-1 rely on tumor cell-intrinsic activation of the cytosolic RNA receptor RIG-I (Fig. 1A). Mechanistically, tumor cell-intrinsic RIG-I signaling induced caspase-3-mediated tumor cell death, cross-presentation of tumor-associated antigen by CD103+ dendritic cells, subsequent expansion of tumor antigen-specific CD8+ T cells, and their accumulation within tumor tissue. Consistently, therapeutic targeting of RIG-I with 5'-triphosphorylated-RNA in both tumor and non-malignant host cells potently augmented the efficacy of CTLA-4 checkpoint blockade in several tumor models. In humans, transcriptome analysis of primary melanoma samples revealed a strong association between high expression of DDX58 (the gene encoding RIG-I), T cell receptor and antigen presentation pathway activity and prolonged overall survival (Fig. 1B). Moreover, in melanoma patients treated with anti-CTLA-4 checkpoint blockade, high RIG-I transcriptional activity significantly associated with durable clinical responses (Fig. 1C). Our preclinical data further demonstrate that tumor cell-intrinsic RIG-I signaling is also an essential pathway for the efficacy of other immunomodulating anticancer treatments including radiotherapy or hypomethylating agents such as 5-azacytidine. We thus identify aberrant tumor cell-intrinsic RIG-I signaling to be a crucial mechanism underlying cancer resistance to checkpoint inhibitor-based and other immunotherapies. These data have immediate translational potential as a RIG-I agonist for human application has been tested in phase I/II clinical trials with local administration in solid tumors and lymphomas (NCT03065023). Intratumoral RIG-I gene expression may not only serve as a biomarker to select patients that will likely benefit from anti-CTLA-4 therapy, but clinical RIG-I targeting in patients may also increase overall response rates of checkpoint inhibitor-based immunotherapy of malignancy including lymphoma.
Figure 1. (A) Wild-type (WT) mice were bilaterally challenged with either WT or RIG-I-deficient (RIG-I-/-) B16.OVA melanoma cells.Recipients were repeatedly treated with anti-CTLA-4. Some mice were additionally injected with the RIG-I ligand 3pRNA into the right-sided tumor. Overall survival of treated mice bearing WT or RIG-I-/- B16.OVA tumors. (B) Overall survival in 456 TCGA melanoma patients by expression of DDX58 (RIG-I) in tumor samples. (C)DDX58 (RIG-I) expression in tumor samples from 18 patients with durable clinical response to anti-CTLA-4 treatment versus non-responders. Date give values from individual patients + geometric mean.
van den Brink:Seres Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Flagship Ventures: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Evelo: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria; Therakos: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Merck & Co, Inc.: Consultancy, Honoraria; Acute Leukemia Forum (ALF): Consultancy, Honoraria; Magenta and DKMS Medical Council: Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics: Other: Licensing.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal