Introduction
The use of factor VIII (FVIII) replacement products enables comprehensive management (prophylaxis, acute bleed control, and perioperative hemostasis) of patients with severe hemophilia A. Prophylaxis with standard half-life FVIII replacement therapies requires frequent administration, and low FVIII activity levels between infusions lead to an increased risk of bleeds. FVIII replacement products that achieve optimal bleed protection with once-weekly dosing intervals remain an unmet need for people living with severe hemophilia A.
BIVV001 (rFVIIIFc-VWF-XTEN) is a novel FVIII therapy composed of single-chain FVIII, the Fc domain of human immunoglobulin G1, the FVIII-binding D′D3 domain of von Willebrand factor (VWF), and 2 XTEN polypeptides. BIVV001 is designed to be a next-generation FVIII therapy that circulates independently of endogenous VWF, thereby breaking the VWF-imposed half-life ceiling.
Single-dose BIVV001 was well tolerated and provided sustained FVIII activity in a first-in-human trial (Konkle et al, Blood, 2018). Here, we report final data for an open-label Phase 1 trial to assess the safety, tolerability, and pharmacokinetics (PK) of repeat dosing with BIVV001 in subjects with severe hemophilia A (<1 IU/dL [<1%] endogenous FVIII) (EudraCT No: 2018-001535-51).
Methods
Eligible subjects were 18-65 years of age, had severe hemophilia A, and ≥150 exposure days to prior FVIII products. After screening and washout, subjects received 4 once-weekly doses of BIVV001 (Days 1, 8, 15, and 22) at either 50 IU/kg (Cohort 1) or 65 IU/kg (Cohort 2). The safety observation period extended for 28 days after the last dose of BIVV001. Primary endpoints were the occurrence of adverse events and clinically significant abnormalities in laboratory tests, including inhibitor development. Secondary endpoints were PK parameters derived from FVIII activity evaluated using a one-stage activated partial thromboplastin time clotting assay. PK blood samples were collected immediately before BIVV001 infusion on Days 1, 8, 15, and 22 and at multiple times after dosing on Days 1 and 22.
Results
All subjects enrolled in Cohort 1 (n=10) and Cohort 2 (n=14) completed the study. Mean (range) age of subjects was 35 (25-55) years for Cohort 1 and 41 (24-58) years for Cohort 2. BIVV001 was well tolerated. No inhibitor development to FVIII was detected, and there were no events of hypersensitivity or anaphylaxis reported.
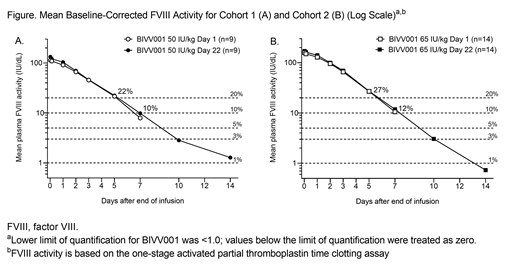
Baseline-corrected PK data were available for 9 subjects in Cohort 1 and all subjects in Cohort 2. Consistent with the single-dose study, the geometric mean (range) half-life for 50 IU/kg and 65 IU/kg BIVV001 was 41.3 (34.2-50.1) hours and 37.3 (28.9-43.8) hours, respectively. After 4 weekly doses of BIVV001 (Day 22), geometric mean (range) area under the activity-time curve from hour 0 over the dosing interval (AUC0-tau) and maximum concentration at steady state (Cmaxss) of BIVV001 were 8290 (5810-10,300) hr × IU/dL and 131 (96-191) IU/dL for Cohort 1 and 11,200 (7040-15,800) hr × IU/dL and 171 (118-211) IU/dL for Cohort 2, respectively. Mean (standard deviation) FVIII activity immediately prior to the final dose of BIVV001 (Ctrough) was 9.9 (2.8) IU/dL in Cohort 1 and 11.7 (5.5) IU/dL in Cohort 2. The mean (range) Day 22-Day 1 accumulation index was 1.07 (1.03-1.11) for Cohort 1 and 1.05 (1.02-1.08) for Cohort 2. At 5 and 7 days after the final BIVV001 infusion, mean steady-state FVIII activity was 22% and 10% for Cohort 1 and 27% and 12% for Cohort 2, respectively (Figure). Geometric mean (range) incremental recovery after the first dose of BIVV001 was 2.3 (1.6-2.8) IU/dL per IU/kg for Cohort 1 and 2.4 (1.6-3.3) IU/dL per IU/kg for Cohort 2.
Conclusions
Four weekly infusions of 50 IU/kg or 65 IU/kg BIVV001 were well tolerated with no identified safety concerns. FVIII activity levels were sustained and nonaccumulating between doses. By breaking through the VWF-imposed half-life ceiling, BIVV001 prophylaxis may lead to more optimal, extended protection against bleeds for patients with severe hemophilia A than standard FVIII therapies. These results support the continued development of BIVV001 in a Phase 3 clinical trial program.
Lissitchkov:Roche: Consultancy, Equity Ownership, Honoraria, Speakers Bureau; Sanofi: Equity Ownership, Research Funding; Bayer: Consultancy, Equity Ownership, Honoraria, Other: Principal investigator for clinical trials, Research Funding; Sobi: Consultancy, Equity Ownership, Honoraria; Shire: Consultancy, Equity Ownership, Honoraria, Speakers Bureau; Octapharma: Equity Ownership, Research Funding. Rice:Sanofi: Employment. Katragadda:Sanofi: Employment. Willemze:Sanofi: Employment. Benson:Sanofi: Employment. Knobe:Sanofi: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal