Introduction: The landmark VE-BASKET trial demonstrated that Erdheim-Chester disease (ECD) patients with the BRAF-V600E mutation can be effectively treated with vemurafenib 1920 mg/day. However, all patients required dose reductions due to adverse effects. The efficacy of low dose BRAF-inhibitors is not well established in ECD. Further, as Langerhans cell histiocytosis (LCH) also harbors BRAF-V600E mutations in 50-60% of patients, BRAF-inhibitors may be effective in this disease as well. In this study, we evaluated the efficacy of low dose BRAF-inhibitors (vemurafenib and dabrafenib) in the treatment of ECD and LCH.
Methods: We conducted a retrospective study of ECD and LCH patients who were seen at our institution from January 1998 to July 2018. All patients had a diagnosis of ECD and LCH determined by clinical criteria in conjunction with histopathologic findings. Based on the standard doses approved for malignant melanoma and ECD, patients were categorized into the low dose BRAF-inhibitor group if they were treated with vemurafenib < 960 mg/day or dabrafenib ≤ 150mg/day at any point in time. We used a simple response criteria that defined clinical progressive disease (PD) as worsening of symptoms attributed to ECD/LCH or radiologic PD as a progression or worsening of proven or suspected lesions due to ECD/LCH. The minimum duration for symptom improvement to be considered a response was set at 3 months.
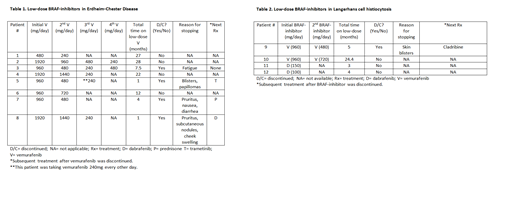
Results: A total of 89 ECD patients and 186 LCH patients were identified. Within the ECD cohort, 24 of 44 (55%) patients who were tested had the BRAF-V600E mutation. Eight patients were included in the low dose BRAF-inhibitor group. The median age at diagnosis was 57 years (range 37-74) and 5 (63%) were male. The most common areas of involvement included bone (88%), cardiovascular system (63%), kidneys (63%), and brain parenchyma (50%). The median time of follow up was 66 months (range 23-165) and the median time on low-dose BRAF-inhibitor was 10 months (range 1-27) [Table 1]. Three patients had a starting dose of vemurafenib 1920 mg/day, 4 had 960 mg/day, and 1 had 480 mg/day. All patients required dose reductions and 50% of the patients ultimately discontinued vemurafenib due to side effects. Side effects included fatigue, pruritus, nausea, facial swelling, blisters, papillomas, and/or subcutaneous nodules. Four patients were able to remain on low-dose vemurafenib for a median follow up time of 24.5 months (range 12-28) with ongoing response and no signs of clinical or radiologic PD.
Within the LCH cohort, 18 of 31 tested (58%) patients had the BRAF-V600E mutation. Four patients were included in the low dose BRAF-inhibitor group. The median age at diagnosis of this cohort was 43 years (range 34-69) and 2 (50%) were male. Areas of involvement included bone (100%), brain parenchyma (hypothalamus/optic stalk and pons; 50%), and skin (25%). The median time of follow up was 31 months (range 21-46) and the median time on low-dose BRAF-inhibitor was 4 months (range 3-24) [Table 2]. Two patients had a starting dose of vemurafenib 960mg/day. The patients with brain parenchymal involvement had a starting dose of dabrafenib 150mg/day or 100mg/day. All patients taking vemurafenib 960mg/day required dose reductions and one patient discontinued treatment due to skin blistering in her feet. In the patients taking dabrafenib, no side effects have yet been reported. 3 patients had an ongoing response and did not have clinical or radiologic PD. Patient #10 however, had clinical and radiologic PD after being on vemurafenib 720mg/day for 22 months.
Conclusion: Our study suggests a potential role for lower doses of BRAF-inhibitors in ECD and LCH patients harboring BRAF-V600E mutations. Dabrafenib was found to be particularly efficacious and well-tolerated in LCH involving the brain parenchyma. However, patients who undergo dose reductions should be closely monitored due to the risk of disease progression. Careful balance of toxicities and efficacy is needed for optimizing patient outcomes with targeted therapies.
Bennani:Adicet Bio: Other: Advisory board; Kite Pharma: Other: Advisory board; Purdue Pharma: Other: Advisory board; Purdue Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board; Purdue Pharma: Other: Advisory board; Adicet Bio: Other: Advisory board; Kite Pharma: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Adicet Bio: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Kite Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board; Seattle Genetics: Other: Advisory board.
Vemurafenib and dabrafenib for Langerhans cell histiocytosis. Vemurafenib dosage for Erdheim-Chester disease is less than the approved dose of 960mg every 12 hours.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal