Background: The incorporation of crossover in randomized controlled trials is accepted as an ethical obligation, especially in cancer clinical trials. The more common type of crossover is unidirectional, which allows patients assigned to the control arm (either placebo or standard of care) to switch to the experimental arm. In contrast, bidirectional crossover includes the switching of patients from the experimental arm to the control arm. Integrating crossover into the initial study design may have advantages in patient recruitment, and incorporating crossover after initial positive results fulfills ethical requirements. However, crossover can also contribute to confounding major endpoints of studies, such as overall survival or the second progression-free survival interval. For this reason, it is important to investigate and identify potential trends of crossover in clinical trials testing novel therapies. The development of new treatments for hematologic malignancies is promising, and as such, makes these trials susceptible to crossover. Trends in crossover of clinical trials for hematologic malignancies have not yet been studied.
Methods: Data about cancer clinical trials were extracted from clinicaltrials.gov. The search query was limited to completed phase III studies in adult populations. Location was limited to the United States. No date range or funder type was specified. The following search query was used: recruitment status: "completed;" age: "adult (18-64)," "older adult (65+);" sex: "all;" country: "United States;" study phase: "phase 3." These criteria were then applied in individual searches for clinical trials related each hematologic malignancy. The following terms were used in the "disease" search field: "acute lymphoid leukemia," "acute myeloid leukemia," "multiple myeloma," "lymphoma," "chronic myeloid leukemia," and "chronic lymphocytic leukemia." Repeat clinical trials obtained through separate searches were excluded. Studies were then excluded if they were not randomized controlled trials (RCTs) with the primary purpose of treatment, and if they did not test cancer-related interventions.
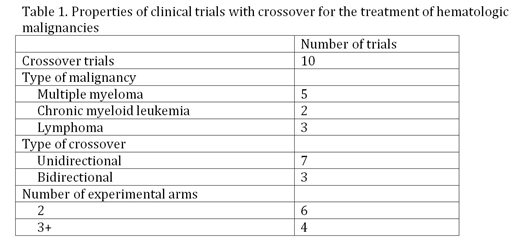
Results: A total of 184 unique clinical trials were identified, of which 156 were RCTs with the primary purpose of treatment. Only 149 trials tested cancer-related drugs, biologicals, or procedures. Of those, 60 trials contained 2 experimental arms, and 89 trials contained 3 or more experimental arms. 6 of the 60 (10%) 2 arm trials permitted crossover, and 4 of the 89 (4.5%) 3+ arm trials permitted crossover, for a total of 10 trials with crossover. The start dates of these trials ranged from 2000-2012. 3 trials (30%) were bidirectional, and 7 (70%) were unidirectional. The identified clinical trials with crossover belonged to only 3 of the original 6 hematologic malignancies included in the original search query: multiple myeloma, lymphoma, and CML (Table 1).
Conclusions: The proportion of identified clinical trials with crossover to those without is extremely small. Crossover in clinical trials studying hematologic malignancies does not appear to be a widespread practice. Multiple myeloma contains the most trials with crossover compared to other hematologic malignancies. Even though statistical approaches to mitigate confounding exist, crossover can still skew accurate reporting of the impact of experimental therapies on overall survival. Examining trends among the trials with crossover will hopefully lead to improved correction methods and better estimates of treatment benefit.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal