Introduction: It has previously been reported that there is a disparity in sex ratios (as well as ethnicity and education level) in subjects enrolled onto clinical trials for investigational medical products (CTIMPs) in oncological trials. This could have an impact for the individual as some reviews have suggested patients on clinical trials have better outcomes than those treated off trial. However, importantly this may have an impact on the analysis of the efficacy, adverse events and recommended dosing of the IMP under investigation due to sex differences in disease biology and drug metabolism. Given the significance of these potential differences, the FDA has established an Office of Women's Health with the specific mandate of ensuring women are represented adequately in trials. The sex ratios of subjects enrolled onto trials for hematological malignancies to the best of our knowledge has not been reviewed previously. We sought to analyse whether there was a difference in enrolment of men and women onto CTIMPs for hematological malignancies in all abstracts presented at the American Society of Haematology Congress in 2018.
Methods: All abstracts that were presented either in oral or poster format at ASH in 2018 were accessed online and reviewed. Only abstracts that were reporting prospective clinical trials involving investigational medical products for hematological malignancies in adult patients were included in the analysis. The significance of the observed percentage of female subjects was analysed by Chi square with the null hypothesis (expected percentage) stated.
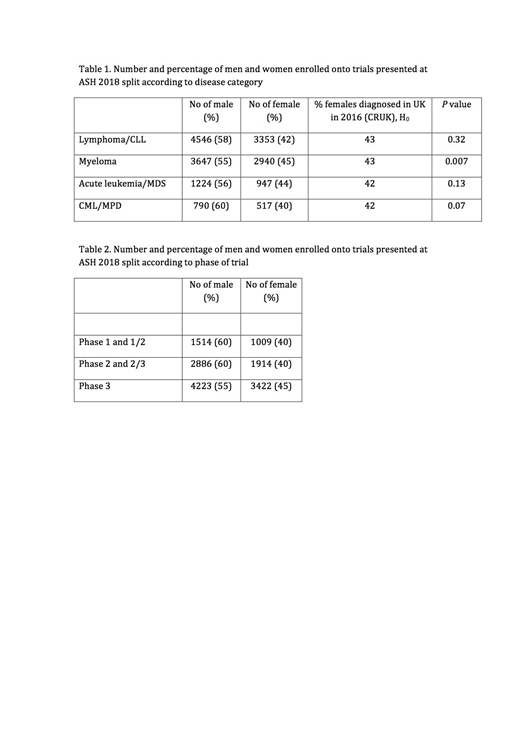
Results: Of all abstracts reviewed, 398 were identified that fulfilled the criteria. If trial names or NCT numbers were specified, these were recorded and 16 were identified as "duplicates", abstracts reporting the same trial or different parts of the same trial and so if applicable only one abstract was included in the analysis. 204 abstracts reported the number of men and women enrolled onto the trial. In total there were 11,056 men (57%) compared to 8243 women (43%) on trial (p<0.0001, H0=50%). The differences according to disease group and phase of trial where stated are shown in the tables. More men were enrolled onto trials then women in all disease groups (Table 1). This was compared to the expected ratio based on the national incidence of all new diagnoses made in the UK in 2016 (www.cancerresearchuk.org, accessed July 2019). In all disease categories, the ratio was as expected apart from trials for myeloma where there was a statistically significant ratio in favour of women being enrolled. Table 2 categorises the percentage of men and women enrolled according to phase of trial. The proportions in these categories were not equal, with the proportion of women entering early phase trials significantly lower than phase 3 trials (p<0.0001).
Conclusions: There was a significant difference in the number of men and women enrolled onto trials for IMPs in hematological malignancies that were presented at ASH 2018. The skew towards male subjects was consistent across disease groups and phase of trial. However this difference may reflect the underlying incidence of these diseases in men and women in the general population rather than represent a true bias in recruitment. However, the proportion of women recruited to early phase trials was significantly lower than those recruited to Phase 3 trials and the reasons for this difference should be investigated further. The data also highlights that a significant number of trials did not report the male to female ratio in the abstract consequently the dataset is not truly representative. Furthermore, the abstracts that have been reviewed only reflect those accepted for presentation which could be further skewing the data. The monitoring of subject recruitment to clinical trials should be performed at a national and international level by regulatory bodies to ensure that the complete trial population is captured and reflects the demographics of the general population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal