Introduction
Comprehensive, accurate assessment by radiologists of PET and CT data is crucial for effective staging, prognostication, response assessment and clinical research in lymphoma. Clinicians require evaluation of disease burden at important nodal and extranodal review sites, review of anatomical sites where toxicity or complications of treatment might be evident, and measurement of parameters specific to lymphoma assessment such as bulk, metabolic SUV and Deauville Score. Ideally, integrated conclusions from multimodal radiological data on staging and treatment response by the radiologist enable appropriate decisions on management to be made at multidisciplinary team meetings and can improve quality of data for clinical research.
Methods
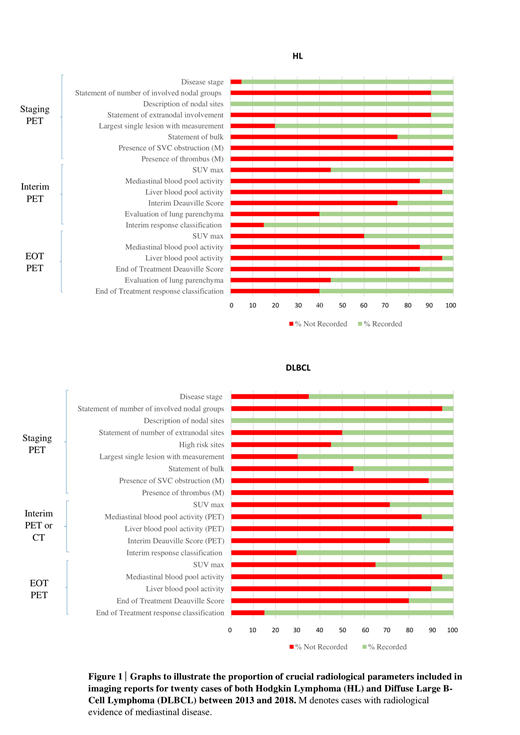
Hodgkin Lymphoma (HL) and Diffuse Large B-Cell Lymphoma (DLBCL) are the two lymphoma subtypes where there is most published evidence for the application of PET and CT. Key PET and CT parameters deemed clinically important in the management of HL and DLBCL at 3 time points - staging, interim and end of treatment - were identified. These parameters are listed in Figure 1. We performed a retrospective review of PET and CT reports at these 3 time points for 20 cases each of HL and DLBCL, over a 5- year period from 2013-2018, to assess the proportion of reports that included each of these key radiological parameters.
Results
Analysis of staging scan reports demonstrated that disease stage was not recorded in 5% of HL reports and 35% of DLBCL reports. 90% of HL reports did not include a statement of the number of involved nodal groups. The involved nodal groups were however described in 100% of reports. 90% of HL reports did not state whether there was extranodal involvement of disease, but extranodal sites were described in 60% of cases. 50% of DLBCL reports did not include the number of extranodal sites. A measurement of the largest single lesion was not given in 20% of HL and 30% of DLBCL reports. In 75% of HL reports and 55% of DLBCL reports, it was not stated whether there was evidence of bulk. With regard to cases where mediastinal disease was noted, 100% of HL and 89% of DLBCL reports had no documentation of whether there was evidence of SVC obstruction. In 100% of these cases for both HL and DLBCL, there was no documentation of whether there was evidence of thrombus.
In terms of PET reports in HL, analysis of interim and end of treatment (EOT) reports demonstrated that SUV max was not given in 45% of interim and 60% of EOT reports. Mediastinal blood pool activity was not given in 85% of interim and 85% of EOT reports. Liver blood pool activity was not given in 95% of interim and 95% of EOT reports. Deauville score was not stated in 75% of interim and 85% of EOT reports. Response classification was not stated in 15% of interim and 40% of EOT reports. With regard to assessment for potential Bleomycin toxicity, the lung parenchyma was not commented on in 40% of interim and 45% of EOT reports.
For DLBCL, SUV max was not given in 71% of interim PET scan reports and 65% of EOT reports. Mediastinal blood pool activity was not given in 86% of interim and 95% of EOT reports. Liver blood pool activity was not given in 100% of interim and 90% of EOT reports. Deauville Score was not given in 71% of interim and 80% of EOT reports. Response classification was not stated in 29% of interim and 15% of EOT reports.
Conclusion
Our data demonstrates retrospective evidence that a significant proportion of imaging reports in HL and DLBCL lack inclusion of radiological parameters crucial to staging, prognostication and response assessment in lymphoma, and required by clinicians to make appropriate management decisions. We therefore propose the implementation of a reporting framework including the parameters outlined in our study, to improve the quality of image reporting in lymphoma and have a positive impact on patient outcomes. Given that lymphoma imaging increasingly involves the use of multiple modalities, we further propose an integrated, standardised radiology reporting framework, under the title 'Specialist Integrated Haematological Malignancy Imaging Reporting', to improve patient outcomes and benefit clinical research.
Iyengar:Abbvie: Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal