Background: Patients ≥55 years of age with rel/ref AML who have failed to achieve complete remission (CR) after standard induction & salvage therapies do not routinely undergo allogeneic hematopoietic cell transplantation (HCT) due to inability to receive myeloablative conditioning and lack of efficacy. With the advent of recently approved targeted therapies (e.g. venetoclax, IDH inhibitors) more patients achieve CR, however durable responses in heavily pre-treated patient populations remain unsatisfactory with very few patients being transplanted. The SIERRA (Study of Iomab-B in Elderly Relapsed or Refractory AML) trial is a prospective, randomized, phase 3 trial designed to address this unmet need. We present the preliminary analysis of the first 50% of patients and analyze the proportion of patients who fail standard chemo and targeted therapies that can then successfully undergo HCT with Iomab-B-based conditioning. Study continues to enroll (N=150), primary end-point durable CR of ≥180 days.
Methods: Eligible patients are ≥ 55 years with rel/ref AML (≥5% blasts), adequate organ function, and related/unrelated 8/8 HLA-matched donors. Patients are randomized (1:1) to receive Iomab-B followed 12-14 days later by reduced intensity conditioning (fludarabine(FLU)/TBI) and HCT, or to conventional care (CC). CC patients may receive investigator's choice of salvage therapy, including newly approved targeted agents such as venetoclax, IDH Inhibitors and may proceed to standard HCT if they achieve CR. If patients do not achieve CR, the study allows cross-over (CO) to Iomab-B with HCT.
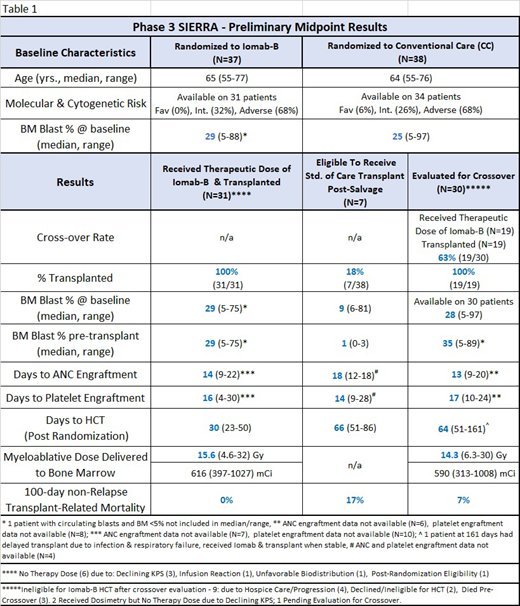
Results: Preliminary data are available from the first 75 patients enrolled on the SIERRA trial. Median age of 64 years (range: 55-77). All patients had active measurable disease (Table 1). Patients in this trial were heavily pretreated with 85% failing ≥2 induction therapies and 33% failing targeted therapies. Molecular and cytogenetics was 68% adverse risk. (NCCN, v1.2018) Following therapeutic dose of Iomab-B as a single agent, there was significant reduction of circulating blasts by day 3 (99%, p<0.0001), and peripheral blasts became undetectable by day 8 prior to FLU. All patients transplanted on the Iomab-B arm with data available showed median days to engraftment of ANC at 14 (range: 9-22) and platelets at 16 (range: 4-30) (Table 1). After randomization, 82% (31/38) patients in the CC arm failed salvage therapy and did not proceed to standard HCT. Significantly, 32% (12/38) of these patients received targeted therapy. 73% (8/11) of the patients receiving venetoclax with HMA or cytarabine did not adequately respond and did not proceed to standard HCT, 45% (5/11) crossed over to Iomab-B and received HCT. Following standard chemotherapies, 85% (22/26) were evaluated for CO. In total, 63% (19/30) received Iomab-B and HCT. The most common reason preventing crossover was disease progression. All crossover (Iomab-B) patients showed similar engraftment (Table 1). CC patients showed increased incidence of febrile neutropenia with grade 3 prior to CO evaluation or HCT, compared to Iomab-B patients prior to HCT (CC: 34%, Iomab-B: 8%). Iomab-B administration was generally well-tolerated with no grade 4 and 1 grade 3 infusion reaction. There were no Iomab-B-related deaths or 100 day non-relapse transplant related mortality deaths in patients on the Iomab-B arm (Table 1).
Conclusion: 100% of the patients receiving HCT on the Iomab-B arm engrafted, despite a median of 29% BM blasts pre-Iomab-B. 82% of the patients on the CC arm failed to be eligible for standard of care transplant, including a high percentage of those receiving targeted therapies. On the CC arm, 63% of eligible patients crossed over to Iomab-B/HCT with robust engraftment despite a median of 35% BM blasts pre-Iomab-B. The incidence of grade 3 febrile neutropenia was higher in patients treated on the conventional care arm with salvage therapy vs. those treated on the Iomab-B arm prior to HCT. In conclusion, despite advanced age, active disease and heavily pre-treated patients, 68% (50/74) of all patients enrolled in the SIERRA trial were able to undergo HCT after reinduction and targeted conditioning with Iomab-B. These results show significant improvements in the current rates of transplantation in this patient population. This SIERRA trial is currently enrolling. For full study details see www.sierratrial.com or clinicaltrials.gov (NCT02665065).
Gyurkocza:Actinium Pharmaceuticals: Research Funding. Nath:Astellas: Consultancy; Actinium: Consultancy; Daiichi Sankyo: Consultancy. Stiff:Amgen: Research Funding; Gamida-Cell: Research Funding; Incyte: Research Funding; Unum: Research Funding; Cellectar: Research Funding; Gilead/Kite Pharma: Consultancy, Honoraria, Research Funding. Tomlinson:Actinium Pharmaceuticals, Inc: Other: Travel Support. Abhyankar:Incyte: Speakers Bureau; Therakos: Other: Consulting, Speakers Bureau. Hari:AbbVie: Consultancy, Honoraria; Cell Vault: Equity Ownership; Sanofi: Honoraria, Research Funding; Spectrum: Consultancy, Research Funding; Amgen: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Kite: Consultancy, Honoraria. Al-Kadhimi:Seattle Genetics: Other: Stocks; Celldex Biotech: Other: Stocks. Foran:Agios: Honoraria, Research Funding. Orozco:Actinium Pharmaceuticals: Research Funding. Van Besien:Miltenyi Biotec: Research Funding. Sabloff:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees; ASTX: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Actinium Pharmaceuticals, Inc: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi Canada: Research Funding. Kebriaei:Pfizer: Honoraria; Kite: Honoraria; Jazz: Consultancy; Amgen: Research Funding; Pfizer: Honoraria; Kite: Honoraria; Jazz: Consultancy; Amgen: Research Funding. Levy:Takeda (Millennium Pharmaceuticals): Consultancy. Lazarus:Adaptive Biotechnologies: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; Actinium Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Teva Pharmaceuticals: Speakers Bureau; Biosight: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; AstraZeneca: Honoraria, Speakers Bureau; Bristol Myers Squibb: Honoraria, Speakers Bureau; Pluristem Therapeutics, Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Consultancy; Genentech: Speakers Bureau. Giralt:Novartis: Consultancy; Actinium: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy; Amgen: Consultancy, Research Funding; Kite: Consultancy; Miltenyi: Research Funding; Takeda: Consultancy, Research Funding; Johnson & Johnson: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy. Berger:Actinium Pharmaceuticals, Inc: Employment, Equity Ownership. Reddy:Actinium Pharmaceuticals: Employment. Pagel:Pharmacyclics: Consultancy; AstraZeneca: Consultancy; Gilead Sciences: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal