Introduction:
Thrombocytopenia has been reported to predict for poor mobilization and apheresis collection of HPC (Morris CL et al., Br J Haematol 2003; 120(3): 413-23). Collection of HPC in lymphoma and myeloma patients often fails as the result of damage to the marrow following multiple cycles of chemotherapy, which may also result in persistent thrombocytopenia. Plerixafor reduces but does not eliminate collection failures due to poor mobilization. Eltrombopag is an FDA-approved drug for immune thrombocytopenia that has also been shown to be effective in improving all lineages in aplastic anemia. Eltrombopag has recently been shown in a dose-finding pilot study to improve HPC collection in myeloma patients. Here we report its addition to other standard measures to prevent failure to achieve the desired collection goal in myeloma (MM) and non-Hodgkin lymphoma (NHL) patients.
Methods:
We used our previously validated minimum daily predictive formula (Rosenbaum et al., Cytotherapy, 2012; 14: 461-466) to identify thrombocytopenic patients with predicted poor collections of CD34+ cells. These patients had predictions for collection of of <1x 106 CD34+ cells/kg daily for first time collectors and <0.5 x 106 CD34+ cells/kg for those undergoing a subsequent collection attempt. In addition to eltrombopag all patients received G-CSF and plerixafor. Data collected include age, gender, ethnicity, diagnosis, chemotherapy prior to collection, number of lines of chemotherapy prior to collection, dose and duration of eltromopag, peak daily CD34+ cells in blood, and total CD 34+ cells/kg collected. Engraftment data for those who underwent autologous transplant (autoHPCT) were also evaluated.
Results:
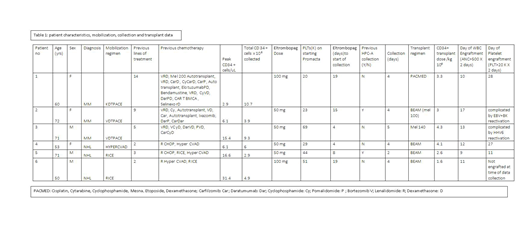
Six non-Hispanic white patients (pts) were identified who met study criteria: median age was 63 years (range 50-72), 3 male/3 female, 3 MM/3 NHL. All were heavily pretreated with chemotherapy: NHL patients had 2, 3 and 2 prior lines of therapy while MM patients had 5, 9 and 14 lines respectively.
The median time from start of eltrombopag to start of collection was 11.5 days (range 4-19). The dose of eltrombopag was 50 mg daily (4 pts) and 2 pts needed dose escalation to 100 mg daily for successful collection. The median peak daily CD34 + cells from peripheral blood during collection was 10.75/uL (range 2.1-31.4). The mean predicted minimum daily CD 34 + cell collection totaled 4.7x 106 (range 3.6 - 6.8x106) and mean total CD 34+ cells actually collected was 6.28x106 (range 3.9-10.7x106). The median days needed for collection was 4 days (range 2-5). Six patients continued to transplant, with regimens of melphalan (MEL) 140 mg/m2 (1 MM), PACMED (1 MM), BEAM (MEL100 mg/m2, 1 MM) and BEAM (3 NHL). The median number of CD34+ cells/kg infused for transplant was 3.15 (range 1.6-4.3x106; mean 3.15). Mean day of WBC engraftment post-transplant was day 12 (range 9-17). Mean day of platelet (PLT) engraftment was day 22 (range 11-27) for 3 pts. Of the other 3, 1 had not engrafted at the time of data collection with a follow up of 19 days and 2 had PLT engraftment complicated by viral reactivation, of whom 1 was given a boost.
Conclusions:
Eltrombopag can be used successfully in NHL as well as MM patients, together with G-CSF and plerixafor, for mobilization and collection of HPC in heavily pretreated pts who are persistently thrombocytopenic prior to starting mobilization and who are predicted to be poor mobilizers. WBC engraftment after auto HPCT was not delayed relative to our historical data, but PLT engraftment was delayed in some cases, which is not uncommon in this patient population where extensive previous therapy has damaged the stem cell niche.
van Rhee:Takeda: Consultancy; Sanofi Genzyme: Consultancy; Castleman Disease Collaborative Network: Consultancy; EUSA: Consultancy; Adicet Bio: Consultancy; Kite Pharma: Consultancy; Karyopharm Therapeutics: Consultancy.
Eltrombopag is FDA approved for immune thrombocytopenia. In this study, it was used for thrombocytopenia thought due to previous chemotherapy
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal