Background:Low weight children can sometimes be selected as donor in HSCT. Donor with thalassemia minor has low MCV, which affects peripheral blood stem cell (PBSC) harvest. It is worthwhile to explore a harvest program for donors with low body weight and low MCV.
Objective:The aim of this study is to find out an appropriate program of PBSC collection in children with low body weight and low MCV.
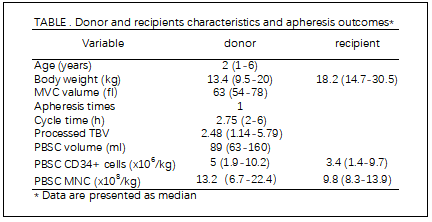
Methods:10 children, with median age of 2 (1-6) years, average weight of 13.4 (9.5- 20) kg and average MVC value of 63fl (54-78)) were hospitalized in our center from October 2018 to July 2019 for stem cell mobilization. Donor weight were less than recipients in 7 cases. 5 donors have 5 kg less than recipient, 3 have 10 kg less and 1 has 18 kg less, respectively. They received G-CSF of 5 mg/kg/d twice a day for 5 days before harvest. 500 ml ACD-A solution and 3000 IU heparin were mixed and given by the ratio of 1 ml mixed anticoagulant versus 24 ml whole blood. Blood-flow was 25-40 ml/min.
Results:More 20/ul CD34+ cells were harvested in 9 cases (90%), and 19.2/ul in 1 case (10%). Median nucleated cells were13.2(6.7-22.4)x108/kg in donor weightand 9.8 (8.3-13.9) x108/kg in recipient weight. The median CD34+ cells were 5 (1.9-10.2) x106/kg in donor weight and 3.4 (1.4-9.7) x106/kg in recipient weight. The coagulation function was normal in reexamination. PBSC were harvested only once in all donors. Average circulating volume was 2.48 (1.14-5.79) total body volume, average harvesting time was 2.75 (2-6) hours, and average PBSC volume was 89 (63-160) ml. Donor peripheral blood calcium and potassium ion concentration showed no obvious abnormality.
Conclusion:Combination of citrate and heparin can be well used to collect PBSC in donors with low body weight and low MCV. All children were well tolerance to the program, which was safe and effective for large volume collection of PBSC.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal