Introduction:
B cell maturation antigen (BCMA) has become a popular research target for multiple myeloma (MM) in chimeric antigen receptor (CAR)-T cell therapy. Up to now, many trials with anti-BCMA CAR-T cells has demonstrated inspiring outcome in patients with relapsed or refractory (R/R) MM. While, cytokine release syndrome (CRS) is a big challenge, it occurs in almost 76% of the patients, and can be fatal if not managed well. However, the pathophysiology of CRS is not so clear, and dynamic changes are ignored. Here, we deeply analyze the dynamic changes of various cytokines in different stages of CRS, trying to find the cytokines closely related to CRS and looking for the target proteins that might be used to control CRS.
Methods:
28 patients with R/R MM were enrolled and got treatment with informed consent in Ruijin Hospital, First Affiliated Hospital of Nanjing Medical University and Changzheng Hospital from April 3, 2017 to October 8, 2019. All patients received anti-BCMA CAR-T cells infusions at doses of 0.05~2.78×106 CAR+ T cells/kg. Criteria previously reported by the CARTOX working group were adopted for the grading of CRS. Genomic DNA was isolated from whole blood for CAR-T cells detection by qPCR. 61 cytokines were assessed in the serum of 25 patients before infusions and at multiple time points after infusions within three weeks (Magnetic LuminexR Assay; R&D Systems). P values were determined using Mann-Whitney U test.
Results:
Within one month, CAR-T cells presented proliferation in all patients tested and the median peak value of CAR+T was 78148 copy number/μg DNA. All patients experienced CRS, which generally occurred at a median of 7 days (range 3-10) after infusions. And the median time of fever onset was 8 days after infusions. Of the 28 cases, 46% of patients had grade≥3 CRS.
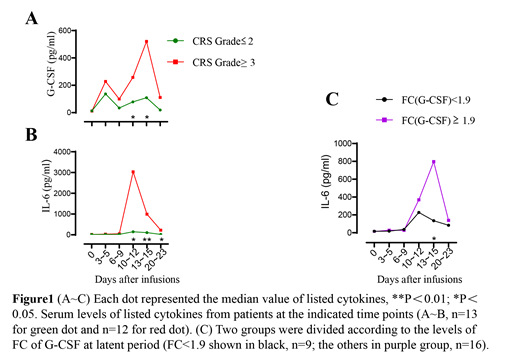
To better understand the dynamic changes in cytokine profile, we chose 6 different time points in each patient which represented baseline period (time before infusions), latent period (day 3~5 after infusions), fever period (day 6~9 after infusions), acute aggravation period (day 10~12 and day 13~15 after infusions), remission period (day 20~23 after infusions). Levels of many cytokines were increased remarkably after treatment. The peak fold-change (pFC) over the baseline was calculated for each cytokine in each patient, IL-6 ranked first with a median pFC of nearly 92 times, followed by Granzyme B, IL-10, G-CSF. And IL-6 was the most closely associated cytokine with Grade≥3 CRS (P=0.005) among all cytokines. For exploring the early initiation cytokines for CRS, FC over the baseline of all the cytokines were analyzed. Cytokines with a median FC of over 2 times at latent period were G-CSF, GM-CSF, Granzyme B and IL-1β, which were in sharp contrast to others for example IL-6. The levels of these cytokines in Grade≥3 CRS were higher than that of Grade≤2 CRS, especially at acute aggravation period with significant difference (Fig.1A~B). Meanwhile, high levels of IL-6 in which group the FC of G-CSF was over 1.9 at latent period may indicate that G-CSF had a warning effect on the rise of IL-6 (Fig.1C).
Conclusions:
We have conducted the largest protein chip screening for exploring CRS due to CAR-T cell therapy so far. As the CAR-T cells expanded in the body, patients began to experience stress and developed CRS in varying degree. IL-6 exhibited the largest median peak FC and highest correlation with severe CRS (Grade≥3 CRS), all of these laid the foundation for the use of tocilizumab (IL-6 receptor antagonist) to control CRS. We also speculated G-CSF may be used as an early CRS indicator or target for early intervention, but more in-depth mechanism exploration is needed to support and testify.
Xu:National Natural Science Foundation of China: Other: Grants; Shanghai Rising-Star Program: Other: Grants; Shanghai Excellent Youth Medical Talents Training Program: Other: Grants.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal