Introduction:
Multiple myeloma (MM) remains an incurable malignancy originating from plasma cell. Chimeric antigen receptor T (CAR T) cells have been used for treating various malignancies and have proven very efficacious especially for some hematologic malignancies. Pre-clinical and phase 1 studies of CAR T-cells designed against various molecular targets in MM patients have demonstrated encouraging anti-tumor activity. With the emerging data and success of antibody based immunotherapy, questions about the clinical efficacy and safety of CAR T-cells continue to emerge. This study aims to provide a systematic review of CAR T-cell therapy in MM through systematic review offering a reference for future research.
Methods:
On 03/28/19, we performed a search for published studies on CAR T cell therapy in MM using the following databases: Embase, Web of Science, Biosis and Scopus. Following PRISMA guidelines, search results were screened by two independent reviewers. Clinical studies with safety and efficacy data were included in the analysis. In vitro and animal studies and abstracts with duplicate data were excluded.
Results:
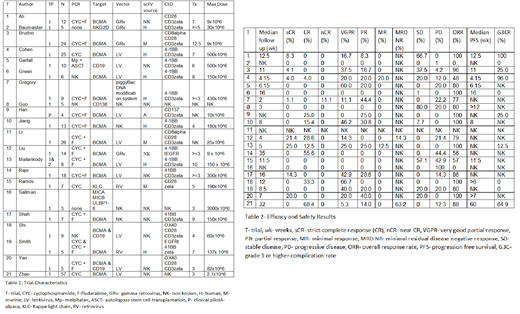
Our literature search produced 1604 results and 21 studies were included in the final review. Of these, 7 were complete manuscripts. Publications were from 2016-2019. 1 was a clinical pilot study, 20 phase 1 trials and 1 study extending to phase 2 with unpublished data. Reported CAR-T cell targets were BCMA (trials [t]=14), CD138 (t=1), CD19 (t=1), Kappa light chain (t=1), multi ligand (t=1), NKG2D (t=1) and 2 studies combining anti BCMA and CD19 CAR T cell infusion. Viral vectors were gamma retrovirus (t=4), lentivirus (t=10), standard retrovirus (t=2), 1 trial without a viral vector and 4 with unknown viral vector. Source of single chain fragment variants were human (t=4), murine (t=3), alpaca (t=1) and 13 unknown. Number of treated MM patients evaluable for efficacy were 4-57. Median prior lines of therapy were 3-12.5 with 3 studies not reporting prior lines of therapy.
Pre conditioning treatments were cyclophosphamide (t=4), cyclophosphamide and fludarabine (t=12), high dose melphalan and autologous stem cell transplantation (t=1), 1 trial without preconditioning and 3 with unknown pre conditioning regimen. Dose of CAR-T cell administered ranged from 2.1x10^6-3x10^9. Median follow up time was 2-35 weeks. Overall response rate was 0%-100%.
Individual response rate was as follows: strict complete response (CR) 0%-21%, CR 0%-68%, near CR 0%-11%, partial response 0%-75%, minimal response 0%-20%, stable disease 0%-100%, progressive disease 0%-100%. Only two studies reported minimal residual disease negative response of 14%-63%. 11 studies reported median progression free survival (PFS) of 4.15 weeks-15 months. 10 studies reported number of patients with grade 3 or higher complications with a rate of complication of 0-96%.
Conclusion:
Results of trials using CAR-T cell therapy in MM are promising for heavily treated relapsed MM patients. BCMA has emerged as an appropriate target for CAR T based immunotherapy. Possibility of a longer lasting PFS makes this modality of treatment very desirable. Variability in safety and efficacy data may be related to patient related factors, disease severity, prior lines of therapy and may uniquely be linked with CAR-T cell construct. Larger prospective clinical studies and randomized control trials are needed to further study the role and safety issue with CAR T cell therapy in relapsed and refractory MM.
Anwer:In-Cyte: Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal