Introduction
Multiple myeloma afflicted cells express signaling lymphocytic activation molecule family member 7 (SLAMF7). Elotuzumab; a monoclonal antibody (mAb), targets SLAMF7 and fights myeloma cells by stimulating phagocytic action of natural killer (NK) cells and via antibody‐dependent cell‐mediated cytotoxicity (ADCC) pathway. This study focuses on the clinical utility and tolerability of elotuzumab based combinations in patients (pts) with multiple myeloma (MM).
Methods
Literature search of PubMed, Embase, Clinicaltrials.gov, Cochrane and Web of Science was performed from inception to June 12, 2019 for elotuzumab based combinations in MM patients. Total of 06 phase II and phase III clinical trials were selected for systematic review out of 292 studies.
Results
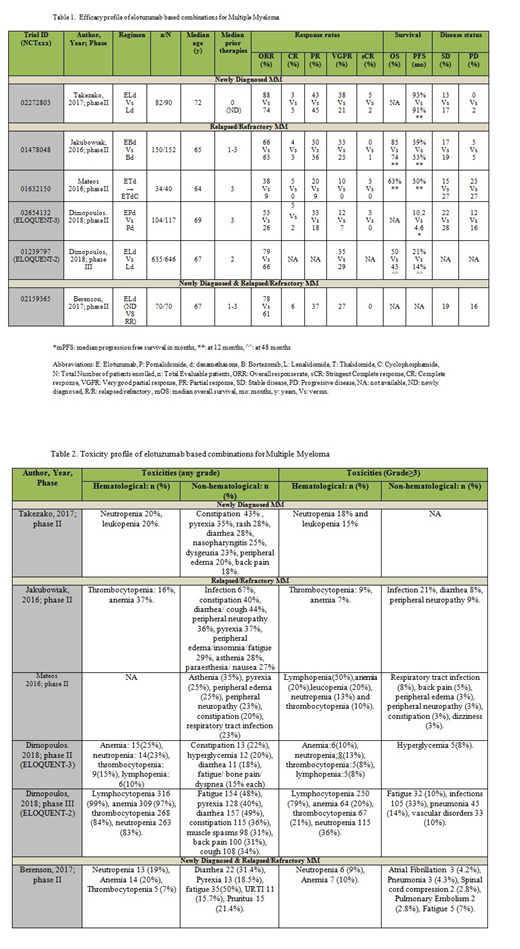
Elotuzumab (E) based combinations were evaluated in 1075 patients (pts) out of 1115 enrolled pts. 119 were newly diagnosed (ND) and 956 were relapsed/refractory (RR) patients.
Newly Diagnosed MM patients: Takezako et al. 2017; in phase II trial, showed overall response rate (ORR) of 88% (complete response [CR] 3% + stringent CR [sCR] 5% + partial response [PR] 43% + very good partial response [VGPR] 38%) with ELd (E-Lenalidomide-dexamethasone) arm vs 74% in Ld arm in 82 ND pts. Progression free survival (PFS) at 1 year (y) was 93% versus (vs) 91%. Grade (G) ≥3 adverse events (AEs) included neutropenia (18%) and leukopenia (15%).
Relapsed/Refractory MM patients: E-Bortezomib(B)-d arm in a phase II trial by Jakubowiak et al. (2016) showed 1 y PFS of 39% vs 33% in Bd arm in 152 RR pts (Hazard ratio [HR]: 0.72; p = .09) with 28% decrease in progression or death with EBd vs Bd arm. Objective response rate (ORR) was 66% (4% CR+ 33% VGPR + 30% PR) vs 63%. OS (overall survival) at 1 y was 85% (EBd) and 74% (Bd) (HR: 0.6). G≥3 AEs were infections (21%), thrombocytopenia and peripheral neuropathy (9% each). Mateos et al. (2016) in phase II trial (n=40) evaluated E-T (thalidomide)-d with or without Cy (cyclophosphamide) in heavily pre-treated RR pts. Pts received Cy if they failed to achieve PR by the 5th cycle or because of progressive disease (PD). Responses were better with ETd vs ETdCy. ORR was 38% (95% CI: 23-54) in ETd vs 9% (95% CI: 0-41) in ETdCy. Entire study yielded median PFS (mPFS) of 3.9 months (mo) (95% CI: 2.79%-9.43%) with 1-y PFS of 30% and mOS of 16.3 mo (95% Cl: 8.87%-25.66%) with 1-y survival rate of 63%. G≥3 AEs were lymphopenia (50%), anemia (20%), leucopenia (20%) and respiratory tract infections (RTI) (8%). Dimopoulos et al. 2018 studied E + pomalidomide (P) + d (EPd) for RR pts with ≥2 prior therapies in phase II ELOQUENT-3 trial (n=117). EPd arm yielded mPFS of 10.2 mo vs 4.6 mo in Pd arm [HR: 0.54 (95% CI: 0.34-0.86; p=0.008)], i.e. 46% lower risk of progression or death in EPd vs Pd arm. ORR was 53% (CR 5% + sCR 3% + PR 33% + VGPR 12%) in EPd vs 26% in Pd arm (odds ratio [OR]: 3.25 (1.49-7.11). G≥3 AEs were anemia (10%), neutropenia and infections (13% each). Phase III ELOQUENT-2 trial randomized 646 RR pts. PFS at 4 y was 21% vs 14% (HR: 0.71, 95% CI: 0.59-0.86; p= .0004), favoring ELd with 29% reduction in myeloma progression or death. With VGPR of 30%, ORR was 79% vs 66 % (ELd vs Ld) with HR: 0.77; 95% CI: 0.62-0.95; p = 0.0176. OS at 4 y was 50% vs 43% (HR: 0.78; 95% CI: 0.63-0.96). G≥3 AEs included lymphocytopenia (79%), neutropenia (36%), anemia (20%) and thrombocytopenia (21%). (Dimopoulos et al, 2018).
Newly Diagnosed & Relapsed/Refractory MM patients: Phase II trial (n=70) by Berenson et al. (2017) studied G≥3 infusion reactions (IRs) using ELd in ND (n=37) and RR (n=33) pts. ORR was 78% (95% CI: 62%‐90%) for ND and 61% (95% CI: 42%‐77%) for RR pts. Other response rates were CR 6%, VGPR 27% and PR 37% in all patients. G3 AEs included anemia in 10% pts (no G3 IRs were seen).
Conclusion: Elotuzumab based combinations showing promising outcomes in terms of ORR, PFS and OS for ND and RR MM patients with tolerable toxicity profile. More clinical studies with elotuzumab in unique and newer combinations need to be tested in prospective trials.
Anwer:In-Cyte: Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal