ABSTRACT
BACKGROUND
Venetoclax blocks the anti-apoptotic B-cell lymphoma-2 (Bcl-2) protein, leading to cell death of Chronic Lymphocytic Leukemia (CLL) cells. Multiple studies have demonstrated the efficacy and safety of venetoclax for treatment of relapsed/refractory or treatment naïve CLL.
METHODS
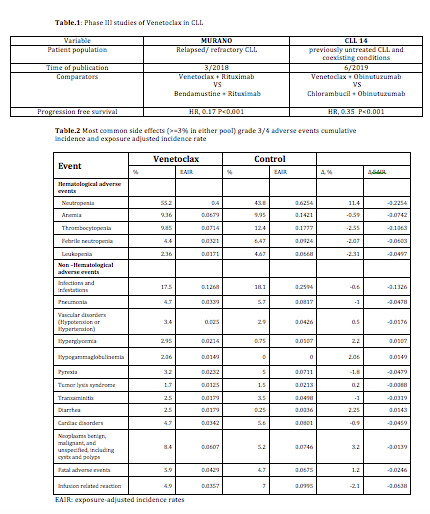
We conducted an integrated safety analysis to characterize the frequency, severity, natural history, and outcomes of adverse events (AEs) with venetoclax versus comparators. Data were pooled from 2 completed phase III randomized controlled studies that had included 406 venetoclax -treated and 402 comparator-treated patients with CLL as a first line in treatment naïve patients and for relapsed/refractory disease (Table.1). Safety analyses included reporting of AEs using crude and exposure-adjusted incidence rates.
RESULTS
The median follow up duration is 25.95months (range: 23.8-28.1 months) for both venetoclax-treated and comparator-treated patients. Discontinuation because of AEs occurred at the same rate in both groups. When adjusted to exposure, hyperglycemia and diarrhea were the only common grade 3 AEs more often reported with venetoclax than with the comparators. The prevalence of common grade 3/4 AEs with venetoclax were <10% apart from neutropenia (55.2%) and infections/infestations (17.5%) though with adjustment to exposure time both of these side effects were more common in comparator arms (Table.2). Grade 3/4 venetoclax associated tumor lysis syndrome happened in 1.7%.
CONCLUSIONS
These results from an integrated analysis support a favorable benefit/risk profile of venetoclax in patients with CLL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal