Introduction
Chemo-immunotherapy (CIT) continues to be an acceptable treatment option in CLL without deletion 17. Patients with early progression (<2 years) have poor response to second line chemoimmunotherapy and a reduced PFS with every subsequent relapse. In developed countries, novel agents like ibrutinib and venetoclax are the standard of care for relapsed CLL and are finding approval as part of frontline therapy as well. In middle to low socio demographic index (SDI) countries, these drugs cost 4 to 40 times the per capita GDP and are unlikely to be affordable to a majority in near future. Therefore, strategies to prolong PFS and the time to next treatment are still relevant in these settings. Lenalidomide has shown promise as maintenance treatment in CLL in two large randomized trials (CLLM1 and CALGB). In this randomized trial, we evaluated the efficacy and safety of individualized, low dose, fixed duration, lenalidomide maintenance versus observation in non-deletion 17p CLL.
Methods
The study period was from January 2018 to June 2019. CLL patients who had achieved at least partial remission (iwCLL response criteria) after frontline CIT were included in the study. Patients with deletion 17p or mutated TP53 were excluded. Baseline and follow-up bone marrow MRD assessment was done using 8-color flow cytometry with a detection threshold of 10-4. MRD was classified into three groups; nil: < 10-4, intermediate: 10-4 to 10-2 and high: >10-2. Patients were randomly assigned to lenalidomide maintenance or observation. Lenalidomide was started at 5 mg every alternate day and escalated to a maximum dose of 10 mg daily in a 20/28 day schedule as per patient tolerance. Maintenance therapy was given for a fixed duration of 6 cycles. PFS was defined as the time from study recruitment to progression or death due to any cause. Haematological toxicity was graded using the iwCLL guidelines for grading of toxicity and other adverse events were graded according to the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE).
Results
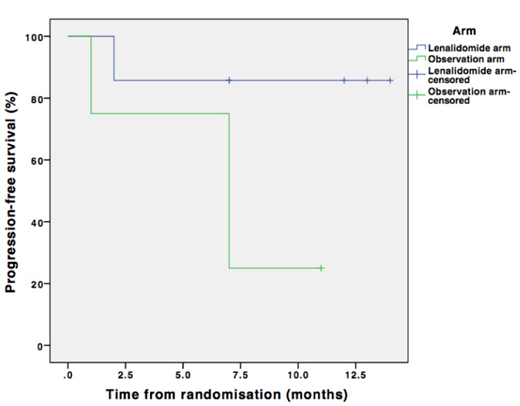
A total of 32 patients were included in the study and randomized to lenalidomide maintenance (n=19) or observation (n=13). The two arms were matched for cytogenetics, comorbidity burden, disease status and MRD at recruitment. At a median follow up of 10.5 months, median PFS was not reached for either arm with no significant difference between the two arms (p=0.338). High MRD at the end of treatment was associated with an increased risk of early progression (p=0.165). Lenalidomide showed PFS benefit in the high MRD subgroup (median PFS 7 months vs. not reached), with a trend towards statistical significance (p=0.067) (see figure). Skin toxicity was the most common adverse event and was more common in the lenalidomide arm (63.2%). Grade 3 neutropenia was reported in only 5.3% patients in the lenalidomide arm. Incidence of grade 3 or more severe infections was not different between the two arms.
Conclusions
Maintenance therapy with 6 cycles of dose-escalated lenalidomide is safe and may afford PFS benefit in patients with non-deletion 17p CLL and high MRD at the end of frontline chemo-immunotherapy. The CLLM1 and the CALGB trials had a fixed ramp up dosing schedule of lenalidomide and reported more adverse events (38% and 71% grade 3/4 neutropenia) compared to our individualized, low dose lenalidomide protocol. The encouraging results of this study warrant further investigation into the role of lenalidomide maintenance in CLL in larger trials, especially in low and middle SDI setting where access to novel agents is limited and is going to remain so in the foreseeable future.
No relevant conflicts of interest to declare.
Lenalidomide maintenance in CLL
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal