Self-renewal is a key feature of the cells that initiate acute myeloid leukemia. To identify the mechanisms involved in PML-RARA (PR)-driven self-renewal, we made use of Ctsg-PR mice, which have PR knocked into the UTR of the first exon of Ctsg. Ctsg-PR drives the expression of PR in myeloid progenitor cells and gives these cells the ability to serially replate in methylcellulose-based colony assays. Most Ctsg-PR mice develop acute promyelocytic leukemia (APL) with an average latency of ~300 days. To identify target genes regulated by Ctsg-PR, we performed single cell RNA-seq (scRNA-seq) on whole bone marrow from young, preleukemic Ctsg-PR mice or age-matched littermates. We identified 959 differentially expressed genes (DEGs) within myeloid progenitors (546 upregulated by PR, and 413 downregulated). Gata2 was identified as a DEG in this analysis, and we confirmed this phenotype with bulk RNA-seq of purified promyelocytes from young, preleukemic WT vs. Ctsg-PR mice. All APLs derived from Ctsg-PR mice also expressed high levels of Gata2.
To identify the immediate-early target genes of PR, we transduced human CD34+ cord blood cells with MSCV-IRES-GFP retroviruses containing PR, a mutant PR with a RARA DNA binding mutation (C88A, known to abolish PR replating), or an empty vector.
ScRNA-seq analysis of these cells after 7 days of ex vivo culture identified 1815 DEGs (1301 upregulated and 514 downregulated by PR) in GFP+ cells expressing PR, compared to GFP+ cells transduced with PRC88A or empty vector. Among the DEGs, Gata2 was upregulated 5-fold in the PR GFP+ cells. Identical short-term retroviral overexpression studies with mouse marrow revealed that PR expression caused an expansion of hematopoietic progenitor cells that overexpressed Gata2. Finally, although normal human promyelocytes do not express GATA2, virtually all primary human APL samples do. Combined, these studies strongly suggest that GATA2 is a target gene of PR, and may therefore play a role in the development of APL.
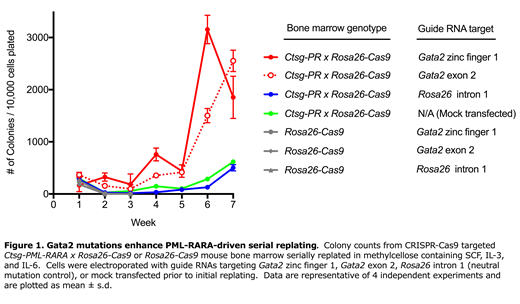
Based on our expression data, we hypothesized that Gata2 inactivation would reduce PR-driven self-renewal. To test this hypothesis, we bred Ctsg-PR mice to Rosa26-Cas9 mice, which ubiquitously express Cas9. Marrow from the resulting Ctsg-PR x Cas9 mice was electroporated with guide RNAs (gRNAs) targeting the zinc finger 1 (ZF1) domain or exon 2 of Gata2, or control loci (Actb intron 5 or Rosa26 intron 1) and serially replated in methylcellulose with SCF, IL-3, and IL-6. CRISPR-Cas9 efficiently induced a wide array of indel mutations at all gRNA target sites. Two days after electroporation, the frequency of Gata2 alleles with indels at the target site ranged from 64% to 93% (n=4); hundreds of different Gata2 indels were generated in each experiment. To our surprise, the Gata2 targeted Ctsg-PR cells replated with dramatically higher efficiency than control locus targeted cells (Figure 1). The enhanced replating efficiency was dependent upon PR, since Gata2 targeted Rosa26-Cas9 bone marrow did not serially replate. Further, cells with Gata2 indels were positively selected for over time. For example, in one experiment, the frequency of a 12 bp deletion in Gata2 (that caused an in-frame deletion in ZF1) rose from an initial variant allele frequency (VAF) of 3% to 70% after 8 weeks of replating. A larger Gata2 deletion was co-selected at a similar frequency, and resulted in the deletion of Gata2 exon 4, which encodes 90% of ZF1. Virtually all of the positively selected cells contained Gata2 indels, demonstrating the competitive advantage of Gata2 loss-of-function mutations in this setting. Control gRNAs did not lead to significant changes in plating efficiency, nor were any indels selected for. Additionally, Ctsg-PR x Cas9 cells with Gata2 indels shifted from a neutrophilic phenotype (Gr1+ CD11b+) to a monocytic one (CD11b+ Gr1-) at late passages. To further investigate the role of Gata2 in Ctsg-PR induced APL, we sequenced the genomes of 16 mouse APLs and found that 2 samples had spontaneous mutations in Gata2 (R330L and N363fs, with VAFs of 74% and 39% respectively). In summary, these data provide evidence that PR positively regulates Gata2, and that Gata2 in turn promotes neutrophilic differentiation of committed, "reprogrammed" myelomonocytic progenitors. Surprisingly, Gata2 appears to contribute to the lineage fate and proliferative capacity of PR-expressing hematopoietic progenitor cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal