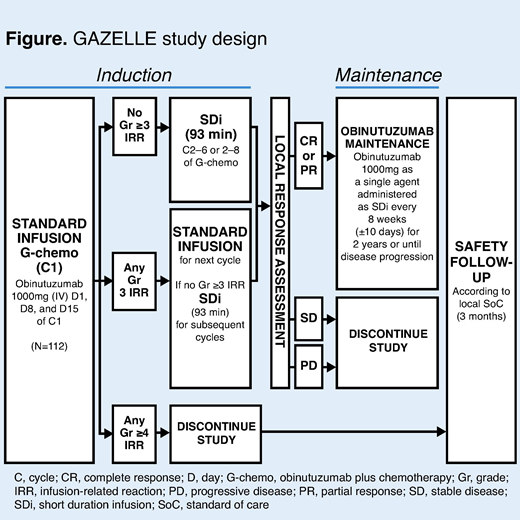
Introduction: In the Phase III GALLIUM study (NCT01332968), obinutuzumab (G) in combination with chemotherapy (G-chemo) demonstrated improved progression-free survival (PFS) and a comparable tolerability profile to rituximab (R)-chemo, in patients with previously untreated follicular lymphoma (FL) (Marcus et al. N Engl J Med 2017). In addition to developing more efficacious treatments for FL, there is also a need for more convenient treatments. The administration of G is associated with infusion-related reactions (IRRs). The incidence of IRRs is greatest with the first infusion and decreases significantly with subsequent infusions (Marcus et al. N Engl J Med 2017). To reduce this risk, an IRR risk-reduction strategy is used in clinical practice. However, this means that administration of G can take approximately 4 hours. Shorter infusion times would yield substantial time savings for patients and benefit outpatient infusion facilities by increasing the number of infusion chairs available during clinic hours and improving nursing efficiency. Previous studies with R have led to the development of a 90-minute short duration infusion (SDi), which is now widely used if the first dose of R is well tolerated. Two Phase II studies have previously evaluated the safety and efficacy of a 90-minute SDi of G. The GATHER study (NCT01414855), in patients with previously untreated advanced diffuse large B-cell lymphoma (Sharman et al. Leuk Lymphoma 2018) and the GATS study in Japanese patients with previously untreated non-Hodgkin lymphoma (Ohmachi et al. Jpn J Clin Oncol 2018) both demonstrated that G can be safely administered as a 90-minute SDi, after the initial cycle (C). Here, we report details of the ongoing GAZELLE study (NCT03817853; MO40597), an open-label, multicenter, Phase IV study investigating the safety of G-chemo, with G administered as a SDi (approximately 90 minutes) from C2 onwards in patients with previously untreated advanced FL, who have received G-chemo at the standard infusion rate without experiencing a grade (Gr) 3 or 4 IRR during C1.
Methods: Patients with CD20-positive, previously untreated stage III or IV FL, or stage II bulky disease (Groupe d'Etude des Lymphomes Folliculaires [GELF] criteria for initiating treatment, Eastern Cooperative Oncology Group performance status [ECOG PS] 0−2, life expectancy ≥12 months) will receive G (1000mg) intravenously on Day (D) 1, D8, and D15 of C1, and on D1 of subsequent cycles (Figure), in combination with chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone [CHOP], cyclophosphamide, vincristine, and prednisone [CVP], or bendamustine at the discretion of the investigator site). The standard infusion rate of G will be administered in C1 (obinutuzumab [Gazyva] prescribing information, 2017). The primary endpoint is incidence of Gr ≥3 IRRs during C2 in patients who had previously received G at the standard infusion rate during C1, without experiencing a Gr ≥3 IRR. For the purposes of the study, an IRR was defined as an adverse event (AE) occurring during or ≤24 hours from the end of G infusion and judged by the investigator as related to the infusion. The rate of G administration at subsequent cycles will be adjusted according to the occurrence and severity of IRRs. Only patients who do not experience a Gr ≥3 IRR during C1 will receive the faster SDi infusion from C2 onwards. If any Gr ≥3 IRR occurs during or after C1 then the next dose of G will be administered at the standard infusion rate. The investigator will decide if the patient can restart with the SDi. Secondary safety endpoints include AEs, time to IRRs, type and duration of Gr ≥3 IRRs and duration of G administration by cycle. Secondary efficacy endpoints include investigator-assessed overall response rate at end of induction, PFS and overall survival at the end of the study, and complete response rate at 30 months. Total length of the study, from screening of the first patient to the end of the study, is expected to be approximately 4 years. It is estimated that 112 patients will be enrolled.
Canales:Novartis: Honoraria; SOBI: Research Funding; F. Hoffmann-La Roche Ltd: Honoraria, Speakers Bureau; iQone: Honoraria; Sandoz: Honoraria; Celgene: Honoraria; Janssen: Honoraria, Speakers Bureau; Gilead: Honoraria; Karyopharm: Honoraria; Takeda: Speakers Bureau. Buchholz:Scripps Health Care System: Employment; Scientific Advisory Committee, Roche/Genetech for the Study of the Abstract: Membership on an entity's Board of Directors or advisory committees. Mason:Abivax: Consultancy. Parreira:F.Hoffmann-La Roche Ltd: Employment, Equity Ownership, Honoraria. Tomiczek:F. Hoffmann-La Roche Ltd: Employment. Hübel:Sanofi: Consultancy, Speakers Bureau; Celgene: Consultancy, Other: Travel, Accommodations, expenses , Speakers Bureau; Hexal: Consultancy; Roche: Consultancy, Other: Travel, Accommodations, expenses , Research Funding, Speakers Bureau; Servier: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal