Background
Waldenström's macroglobulinemia (WM) is a rare indolent cancer. Because of its low incidence, the treatment practices for WM primarily rely on data from phase 2 trials, which often have no consensus as to how to best treat this uncommon disease. The heterogeneity of treatments available can be observed in clinical practice guidelines, which recommend traditional chemotherapies, second-generation proteasome inhibitors, multiagent immunotherapies, and the novel Bruton's tyrosine kinase inhibitor, ibrutinib (IBR). Yet, despite clinical evidence and treatment guidelines recommending multiagent chemoimmunotherapy in first-line (1L) patients with WM, a majority of patients still receive monotherapy, namely chlorambucil in Europe and monotherapy rituximab (R) in the United States. To date, there have been no reports on the real-world treatment practices in 1L of WM since the introduction of IBR. The primary objective of this study is to understand the 1L treatment practices for WM in a nationwide cohort of Veterans treated in the largest integrated healthcare system in the United States, the Veterans Health Administration (VA).
Methods
Using the VA Cancer Registry System and electronic healthcare records, we identified Veterans diagnosed with WM between January 1, 2006, to December 31, 2018. Treatment regimens were classified in accordance with the National Comprehensive Cancer Network (NCCN) guidelines for WM (versions 1.2006, 2.2013, and 2.2019). Eligible patients were followed until loss to follow-up, death or the end of the study observation period (June 30, 2019). The 1L of treatment was examined; with the start date for 1L being the index date. Patients with a cancer diagnosis other than WM and patients who did not receive 1L treatment were excluded from the study.
Results
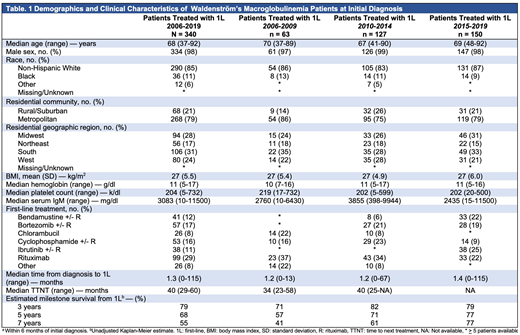
We identified 340 patients who were diagnosed with WM and received a 1L treatment regimen between 2006-2019 in the VA. Median age at diagnosis was 68 years (range: 37-92); 334 (98%) of patients were male. Demographics are further described in Table 1. At diagnosis, the median serum IgM was 3083 mg/dl (range: 10-11500), the median hemoglobin was 11 g/dl (range: 5-17), and the platelet count was 204 k/dl (range: 5-732). A noticeable shift in the adoption of treatments can be observed when comparing treatment practices in patients treated between 2006-2009, 2010-2014, and those treated between 2015-2019. From 2006-2009 the majority of 1L patients received monotherapy with R (23, 37%) or chlorambucil (14, 22%). Between 2010-2014, the majority of patients received monotherapy R (43, 34%), with increasing adoption of bendamustine + R (8, 6%) and bortezomib (27, 21%). Between 2015-2019, IBR became the leading 1L treatment (38, 25%), followed by bendamustine + R (33, 22%), monotherapy R (33, 22%), and bortezomib + R (28, 19%). The estimated survival rate of WM patients treated with 1L was 79% at three-years, 68% at 5-years, and 55% at 7-years.
Conclusions
Our study is one of the first to examine the real-world treatment practices of WM patients treated with 1L after the approval of novel agent IBR. Our results highlight the heterogeneity of treatment options available for WM patients. We also describe the evolution of treatment choices in 1L over the last decade: from chlorambucil and rituximab monotherapy, to ibrutinib, bendamustine, and bortezomib. Retrospective and/or observational studies examining treatments and outcomes in WM patients should take these shifts in treatment practices into consideration. Given the persistent utilization of monotherapy R as a treatment in 1L, despite the superior efficacy of other treatment options such as ibrutinib, bendamustine and bortezomib regimens, our results indicate the need for continued efforts to educate clinicians about the appropriate treatment options available for this rare disease.
Acknowledgments: The study was sponsored by Pharmacyclics
Sauer:University of Utah and SLC VA Medical Center: Employment. Halwani:Genentech, Inc.: Research Funding; Bristol-Myers Squibb: Research Funding; AbbVie: Research Funding; Pharmacyclics: Research Funding; Immune Design: Research Funding; Amgen: Research Funding; Kyowa Hakko Kirin: Research Funding; Seattle Genetics: Research Funding; Takeda: Research Funding; Miragen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal