Introduction
Sirt6, a member of the mammalian sirtuins family, functions as a highly conserved ADP-ribosylase and NAD+ dependent deacylase. Evidence from recent years indicates that Sirt6 is closely related to the occurrence and development of various solid tumors and multiple myeloma (MM). OSS_128167 is a novel small molecular inhibitor specifically targeting Sirt6, and induced chemosensitization in MM cells. Nevertheless, the role of Sirt6 in lymphoid malignancies is still unclear. Herein, we investigated the function of Sirt6 and identified the anti-tumor effects of OSS_128167 in diffuse large B-cell lymphoma (DLBCL).
Methods
Immunohistochemistry (IHC) was conducted to assess the expression of Sirt6 on paraffin-embedded tissues from 70 de novo DLBCL patients and 35 reactive hyperplasia patients with informed contents. Microarray dataset GSE32918 was obtained from Gene Expression Omnibus and survival analysis was performed. Lentivirus vectors either encoding shSirt6, lvSirt6 or empty lentiviral vector were stably transfected into DLBCL cells. shSirt6 transfected or shControl transfected LY1 cell samples were performed RNA sequencing (RNA-seq) analysis, functional enrichment analyses of gene ontology (GO) and gene set enrichment analysis (GSEA). DLBCL cells were subcutaneously injected to SCID-Beige mice to establish xenograft models. All animal experiments were performed in accordance with the principles of the Institutional Animal Care. A p value of less than 0.05 was interpreted as having statistical significance.
Results
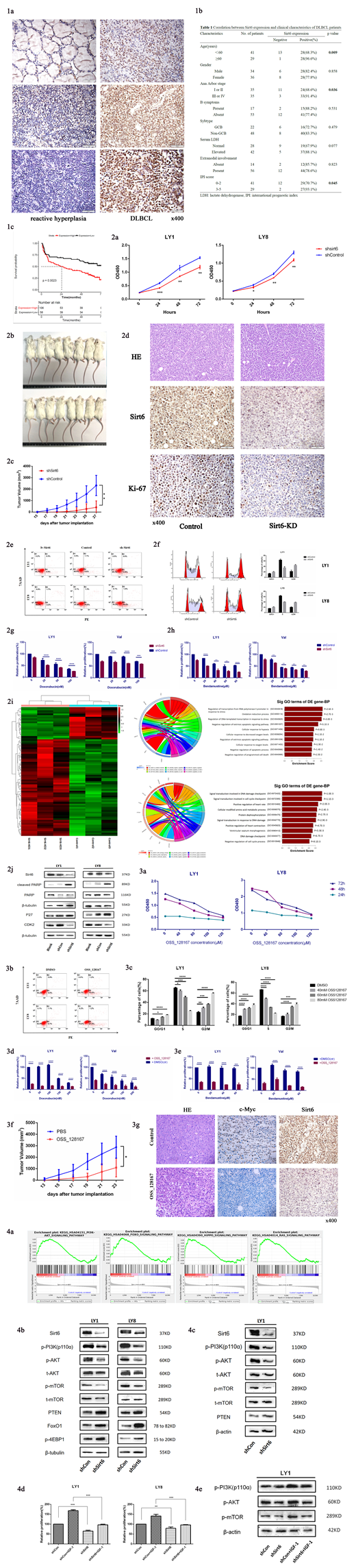
Aberrantly overexpression of Sirt6 was confirmed in DLBCL tissues compared with reactive hyperplasia by IHC staining (Fig 1a). The expression level of Sirt6 was revealed in significant positive correlation with advanced age (p=0.009), Ann Arbor stage (p=0.036) and IPI score (p=0.045, Fig. 1b). Sirt6 high expression was turned up to be correlated with reduced overall survival of enrolled patients according to GSE32918 (n=249, Fig. 1c).
Stable shSirt6 transfected DLBCL cells exhibited growth suppression compared to those transfected with empty vector in vitro (Fig. 2a) and in vivo (Fig. 2b-c). Lower expression level of Ki-67 was confirmed in the xenograft tumor tissues derived from shSirt6 cells by IHC staining (Fig. 2d). Sirt6 knockdown caused enforced apoptosis rates, induced G2/M phase arrest (Fig. 2e-f) and enhanced sensitivity to Doxorubicin (ADR) and bendamustine (Fig. 2g-h). These results were further confirmed in GO analysis according to RNA-seq (Fig. 2i) and WB (Fig. 2j).
The exciting finding was, OSS_128167 obviously decreased cell proliferation (Fig. 3a), induced cell apoptosis (Fig. 3b) and blocked cell cycle (Fig. 3c) in DLBCL cell lines. Addition to ADR or bendamustine with 100µM OSS_128167 exhibited enhanced cytotoxicity in DLBCL cells (p< 0.05; Fig. 3d-e). Xenograft DLBCL mice model were treated with intraperitoneal injection of either OSS_128167 or a control vehicle every two days for 2 weeks. Consistent with our in vitro results, obviously decreased tumor growth were observed in the OSS_128167 group (Fig. 3f). Also, lowly expression level of c-Myc was found in OSS_128167 treated mice (Fig. 3g).
We then investigated the underlying mechanism driving the tumor genesis potential of Sirt6. In accordance with the GSEA analysis, we observed that the phosphorylation of PI3K p110α and its downstream targets, including Akt (Ser473) and mTOR protein, were dramatically decreased in Sirt6 knockdown cells than in the control cells (Fig. 4a-b). In vivo western blotting analysis further confirmed it (Fig. 4c). The Sirt6 deletion-induced reductive of cell viability was only fewer restored by IGF-1 (Fig. 4d).
Conclusion
At present, the function of Sirt6 in tumorigenesis is still controversial. Our findings demonstrated for the first time the oncogenic role of Sirt6 in the pathogenesis of DLBCL. High expression of Sirt6 may serve as a prognostic marker for inferior outcome in DLBCL patients. OSS_128167, a novel targeted inhibitor of Sirt6, exerted excellent anti-lymphoma effects via activating PI3K/Akt/mTOR signaling. In addition, blockade of Sirt6 expression enhanced the sensitivity of DLBCL cells to chemotherapeutic agents. These findings provide mechanistic insights into the oncogenic activity of Sirt6 and highlighted the potency of OSS_128167 for novel therapeutic strategies in DLBCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal