Background Recently, erythropoietin (EPO) was identified as an important regulator of production and cleavage of fibroblast growth factor 23 (FGF23). Since erythroid progenitor cells express high levels of FGF23 and carry the FGF receptor, they are involved in the FGF23 metabolic pathway.
FGF23 is a bone-derived hormone, a key player in phosphate and vitamin D metabolism and regulator of bone mineralization. FGF23 is formed as intact, biologically active protein (iFGF23). Proteolytic cleavage results in formation of an allegedly assumed inactive C-terminal tail FGF23 (cFGF23). FGF23-knockouts or iFGF23 blocking peptides increase erythropoiesis, reduce erythroid cell apoptosis and increase EPO mRNA and circulating EPO. By competitive inhibition, increase in cFGF23 relative to iFGF23 leads to suppression of FGF receptor signaling. (Recombinant) EPO increases the amount of circulating FGF23 (iFGF23 plus cFGF23) and, more importantly, alters the iFGF23/cFGF23 ratio in favor of cFGF23, thereby overcoming suppression of erythropoiesis by iFGF23.
As recently discussed, (van Vuren et al. Front. Physiol. 2019) we hypothesize that EPO-FGF23 signaling plays a role in hereditary hemolytic anemias and is herein related to iron metabolism, inflammation and bone health. We explored this concept further in the current study.
Methods FGF23 was measured in 90 patients and 10 healthy controls of the ZEbRA trial, an observational study at the UMC Utrecht on clinical sequelae and pathophysiology of rare congenital hemolytic (Netherlands Trial Register [NL5189]). An overview of relevant patient characteristics is provided in the Table.
Two FGF23 assays were used: one that detects the C-terminal and therefore quantifies cFGF23 and (full-length) iFGF23 (total FGF23; Immunotopics/Quidel) and one that only detects iFGF23 (Kainos Laboratories).
Chemokines/cytokines were measured simultaneously with a Luminex assay.
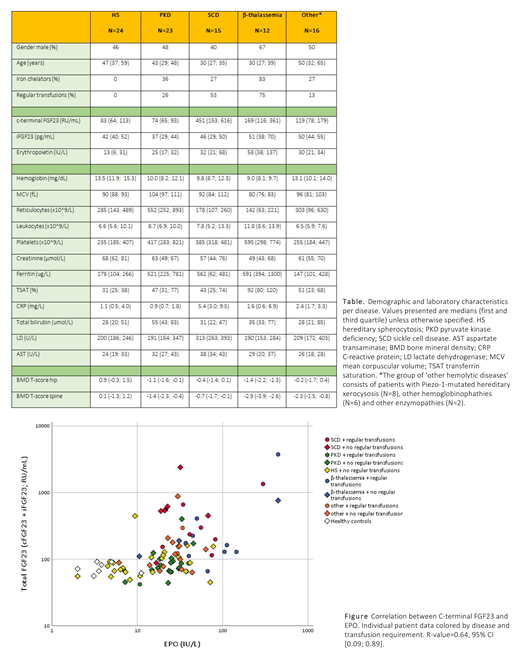
Results We observed a strong correlation between levels of total FGF23 (cFGF23 and iFGF23) and EPO in patients with various hemolytic anemias (r=0.64, 95% confidence interval [0.09; 0.89]) (Figure). As expected, there was neither a correlation between iFGF23 and EPO values nor between iFGF23 and total FGF23. These data are consistent with a decline of the iFGF23/cFGF23 ratio in response to EPO.
iFGF23 and total FGF23 were not correlated to serum calcium, phosphate, creatinine, parathyroid hormone and 25-OH vitamin D (p>0.05). We did not identify a correlation of iFGF23 and total FGF23 with bone health (hip and spine T-scores). EPO was associated with transferrin saturation (r=0.45, 95% CI [0.28; 0.63]) and ferritin (r=0.47, 95% CI [0.06; 0.79]), whereas total FGF23 did not correlate with iron status. The influence of inflammation on the EPO-FGF23 axis was clearly illustrated by higher mean total FGF23 in SCD patients (549 RU/L) compared to other patients (172 RU/L) (95% CI [87; 747]), without a difference in EPO or iFGF23.
We identified a correlation of cFGF23 with CCL4 (r=0.49, 95% CI [0.15; 0.72]) and with sICAM (r=0.54, 95% CI [0.16; 0.75]), markers of endothelial dysfunction and immune players in pulmonary hypertension. (Kato et al. Br J Haematol. 2005; Sweatt et al. Circ Res. 2019)
Conclusion We show for the first time the importance of EPO in FGF23 signaling in hemolytic anemias and investigated its relation with iron, inflammation and bone health.
Absence of a relation between iron and FGF23 suggests that EPO is not simply an intermediary in FGF23 regulation by iron. FGF23 data of SCD patients suggest that inflammation is associated with an additional increase in FGF23 cleavage. Absence of a correlation of FGF23 with bone health might be attributable to treatment for osteoporosis in many patients. Alternatively, systemic FGF23 might underestimate paracrine signaling in the bone.
Additionally, we hypothesize on a contribution of decline in the iFGF23/cFGF23 ratio to vasculopathic complications. Our hypothesis aligns with previous observations that too little or too much FGF signaling is related to endothelial dysregulation and endothelial-to-mesenchymal transition, which contributes to development of pulmonary hypertension. (Chen et al. Cell. Rep. 2012; Morrell et al. J. Am. Coll. 2009)
Current findings open a new area for research and treatment in hemolytic anemias. Therapeutic targeting of the FGF23 pathway might provide an opportunity to intervene in the development of complications.
Glenthøj:Agios Pharmaceuticals, Inc.: Consultancy; Celgene: Consultancy; Alexion: Research Funding; Novartis: Consultancy; Novo Nordisk: Honoraria. van Wijk:Agios Pharmaceuticals: Consultancy, Research Funding; RR Mechatronics: Research Funding. van Beers:Novartis: Consultancy, Research Funding; Agios Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; RR Mechatronics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal