Background: Diagnosing and risk stratifying MDS requires an integrated approach including morphologic, cytogenetic, and molecular genetic assessments to inform treatment decisions and aid prognostication. The WHO classification criteria for diagnosis of MDS were updated in 2016 (Arber DA, et al. Blood. 2016;127:2391-405) with specific recommendations; however, the impact of these updates on clinical practice patterns is unclear. We investigated testing patterns for pts with ND MDS treated in predominantly community- and government-based centers in the Connect® MDS/AML Registry and compared these practices with the WHO 2016 criteria.
Methods: The Connect® MDS/AML Disease Registry (NCT01688011) is a large, US, multicenter, prospective observational cohort study of pts with ND acute myeloid leukemia (AML; aged ≥ 55 years) or MDS (aged ≥ 18 years). Baseline demographics, disease characteristics, and laboratory testing parameters were collected on MDS pts at enrollment to the Registry from December 2013 to March 2019. Pts were classified according to the International Prognostic Scoring System (IPSS) as having lower-risk MDS (LR-MDS; IPSS Low- or Intermediate-1-risk MDS) or higher-risk MDS (HR-MDS; IPSS Intermediate-2- or High-risk MDS). Differences in testing rates at enrollment between LR- and HR-MDS pts were assessed using a chi-square test with P < 0.05 considered statistically significant. Concordance with the recommendations of the WHO 2016 criteria, including frequency of SF3B1 testing, was assessed.
Results: As of March 8, 2019, 694 pts with MDS were enrolled in the Registry; 392 (56.5%) with LR-MDS and 302 (43.5%) with HR-MDS. Median age was 75.0 vs 73.0 years, 66.3% vs 64.9% were male, and 70.6% vs 75.6% were insured by Medicare/Medicaid. There was a significant difference in bone marrow blast enumeration method between LR- and HR-MDS pts; the most common method was manual differential (75.9% vs 72.1%), followed by immunohistochemistry (IHC) (11.5% vs 21.5%) (P = 0.002). The mean (standard deviation) number of cells counted on manual differential was 361.7 (151.3) in LR-MDS pts and 348.2 (163.6) in HR-MDS pts. This was not significantly different between pt groups, but was below the recommended 500 cells. Presence of ring sideroblasts (RS) was more common in LR- than HR-MDS pts (39.8% vs 34.1%; P = 0.02); median percentage of RS was also higher in LR- than HR-MDS pts (25.0% vs 7.5%; P < 0.0001). The majority of pts underwent complete cytogenetic analysis at baseline; 97.4% of LR- and 95.7% of HR-MDS pts. Flow cytometry testing to assess cell lineage was widely done; 97.0% of HR-MDS pts and 93.6% of LR-MDS pts.
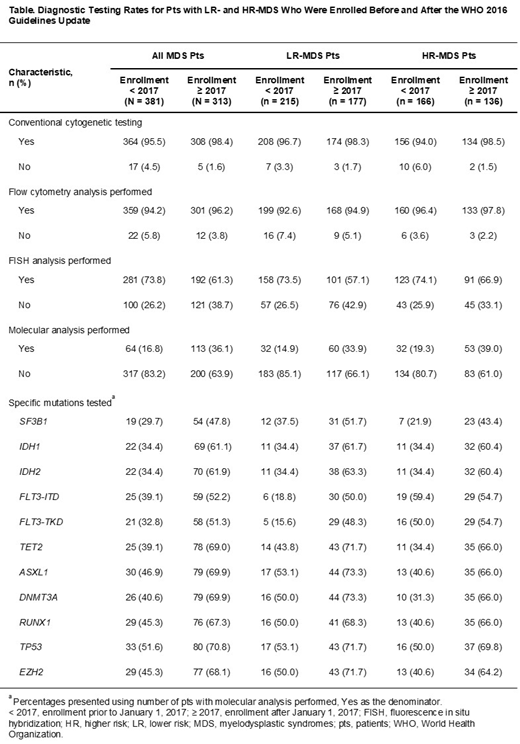
To assess changes in diagnostic testing patterns following publication of the WHO 2016 criteria, LR- and HR-MDS pts were grouped based on their date of enrollment: pts enrolled prior to January 1, 2017, and pts enrolled from January 1, 2017 onward. Molecular testing rates increased in pts enrolled after January 1, 2017, from testing rates seen prior to 2017, in LR- and HR-MDS pts; however, testing rates remained < 40% (Table). Testing rates for specific mutations were higher in pts enrolled after January 1, 2017 than in those enrolled prior to 2017. In parallel with overall testing rates, testing rates for SF3B1 were also higher in pts enrolled after January 1, 2017; however, they remained at approximately 50% in these pts. During this time, rates of fluorescence in situ hybridization testing decreased by 36.1% and 26.0% in HR- and LR-MDS pts, respectively.
Conclusions: In this analysis from the Connect® MDS/AML Registry, increased SF3B1 testing rates were observed following the publication of the WHO 2016 criteria, suggesting uptake of this recommendation by physicians. The increase in testing rates for other specific mutations associated with targeted therapies coincides with the approval of these new therapies by the US FDA. However, further education to increase molecular testing rates for specific mutations, improve prognostication, as well as to ensure the appropriate use of targeted therapies is required. The use of IHC rather than manual differential for blast enumeration, as recommended by the WHO, and the significant difference in enumeration method between LR- and HR-MDS pts, may be due to higher levels of fibrosis in HR pts and poor-quality aspirates rather than a lack of awareness of the recommendations; however, the recommended counting of 500 cells was not performed in all pts.
George:Novartis: Honoraria; Blueprint Medicines: Consultancy; Deciphera: Consultancy; Allakos: Consultancy. Garcia-Manero:Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding; Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding. Grinblatt:Celgene: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy. Komrokji:Incyte: Consultancy; DSI: Consultancy; Celgene: Consultancy; Agios: Consultancy; Janssen: Consultancy; Pfizer: Consultancy; Alexion: Speakers Bureau; Novartis: Speakers Bureau; Jazz: Speakers Bureau. Savona:Boehringer Ingelheim: Patents & Royalties; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sunesis: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Selvita: Membership on an entity's Board of Directors or advisory committees. Scott:Celgene Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Agios: Speakers Bureau. Sekeres:Millenium: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees. Steensma:Stemline: Consultancy; Onconova: Consultancy; Aprea: Research Funding; Arrowhead: Equity Ownership; H3 Biosciences: Other: Research funding to institution, not investigator.; Astex: Consultancy; Summer Road: Consultancy; Pfizer: Consultancy. Flick:Celgene Corporation: Employment. Kiselev:Celgene Corporation: Employment, Equity Ownership. Louis:Celgene Corporation: Employment, Equity Ownership. Nifenecker:Celgene Corporation: Employment, Equity Ownership. Swern:Celgene Corporation: Employment, Equity Ownership. Foucar:Celgene Corporation: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal