Introduction: Refractory anemia with ring sideroblasts (RARS) is a subtype of low risk (LR)-MDS marked by accumulation of abnormal erythroid precursors with iron-filled mitochondria in a ringed pattern around the nucleus. HMAs (azacitidine, AZA; decitabine, DEC) are approved for the treatment of MDS pts in the US and predominantly used for pts with high risk MDS, while Len is approved for treating pts with red blood cell (RBC) transfusion-dependent (TD), deletion 5q (del5q) LR-MDS. As anemic pts with non-del5q LR-MDS often do not respond or eventually stop responding to erythropoiesis-stimulating agents, HMAs and Len are often used in these pts as well. While there is clinical trial and population-based studies supporting clinical effectiveness of HMAs and Len in pts with non-del5q LR-MDS, data regarding activity of these agents in pts with RARS are very limited. Understanding the clinical effectiveness of the agents available in the market in RARS is especially important as new agents for this pt population might enter the market (e.g. luspatercept). We conducted a large population-based study to examine the use of HMAs and Len in lower-risk non-del5q MDS pts, stratifying by RS status.
Methods: Using the Surveillance, Epidemiology, and End Results-Medicare linked database, we assembled a cohort of older adults (66-99 years) diagnosed with non-del5q LR-MDS during 2007-15 with continuous Medicare Parts A&B coverage from 1 year before diagnosis, and Part D coverage from diagnosis, through death or the end of study (12/31/2016), whichever was earlier. Pts with RA, RARS, refractory cytopenia with multilineage dysplasia, refractory neutropenia, and refractory thrombocytopenia were defined as non-del5q LR-MDS pts. We further categorized pts as RS (only RARS) and non-RS LR-MDS. Outcomes included RBC transfusion status and overall survival (OS). RBC TD was defined as having transfusions in at least two non-consecutive weeks during 8-week period, and transfusion independent (TI) was defined as having no transfusion during 8-week period after a history of transfusion use. Kaplan-Meier statistics were used to examine associations between RARS status and OS.
Results: Our cohort included 2167 non-del5q LR-MDS pts with a median age of 79 (interquartile range [IQR]: 74-84) years. Compared with non-RS pts, RS pts (n=640) were more likely to be male, older, have fewer comorbidities and reside in Midwest (Table 1).
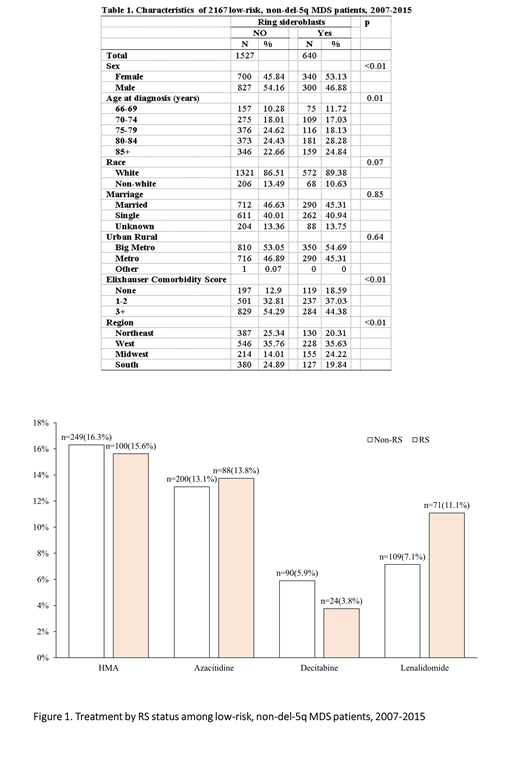
About 16% pts in both groups received HMAs with nearly 80% of HMA users receiving AZA (Figure 1). Median time to initiate HMA was 9.3 (IQR: 3.0-24.4) and 3.9 (IQR: 1.5-12.7) months in RS and non-RS pts, respectively (p<.01). Only 11.1% of RS and 7.1% of non-RS pts received Len with median time to initiation of 8.6 (IQR: 2.3-16.2) months in non-RS pts, and 9.8 (IQR: 3.2-23.1) months in RS pts (p=0.52). A total of 30 RS pts and 45 non-RS pts received both HMAs and Len.
At the initiation of HMAs or lenalidomide, 41.8% of RS and 37.1% of non-RS pts were RBC TD. Among 70 RS pts who used HMA only, 37.1% were TD at initiation; after a median of 13 (IQR 11-26) weeks, 53.8% became TI. Among 204 non-RS pts who used HMA only, 36.8% were TD at initiation; after a median of 15 (IQR 11-19) weeks, 57.3% became TI. Median duration of transfusion independence was 13 (IQR 2-50) and 21 (4-43) weeks, respectively, for RS and non-RS pts who became TI. Among pts who only received Len, 41.5% of RS and 39.1% of non-RS pts were TD, with more than 40% changing from TD to TI in both groups after a median of 20 weeks.
Median OS was 2.8 and 2.1 years for RARA and no-RS pts, respectively (log-rank p<.01). Among RS pts, median OS was 2.6, 3.2, 3.4, and 2.8 years for those who received no treatment, HMA only, HMA and Len, and Len only, respectively (log-rank p=.01). However, among non-RS pts, no significant difference was observed across four treatment groups with median survival of 2.1, 2.0, 2.3, and 1.7 years, respectively (log-rank p=0.17).
Conclusions: In this large, population-based study of older adults with non-del5q LR-MDS, our results suggest that HMAs and lenalidomide are clinically active in pts with RARS. While the RBC transfusion independence rate with HMAs (54%) was somewhat higher than was reported for luspatercept (38%), the median duration appears shorter (13 vs 31 weeks). However, these numbers are limited by small sample size and more research is needed to understand the comparative clinical effectiveness for therapies that target anemia in pts with RARS.
Wang:Celgene Corporation: Research Funding. Zeidan:Cardinal Health: Honoraria; Daiichi Sankyo: Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; Acceleron Pharma: Consultancy, Honoraria, Research Funding; BeyondSpring: Honoraria; Celgene Corporation: Consultancy, Honoraria, Research Funding; Otsuka: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Medimmune/AstraZeneca: Research Funding; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Trovagene: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Research Funding; Jazz: Honoraria; Ariad: Honoraria; Agios: Honoraria; Novartis: Honoraria; Astellas: Honoraria; Seattle Genetics: Honoraria. Podoltsev:Sunesis Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding; Arog Pharmaceuticals: Research Funding; Genentech: Research Funding; Daiichi Sankyo: Research Funding; Kartos Therapeutics: Research Funding; Boehringer Ingelheim: Research Funding; AI Therapeutics: Research Funding; Samus Therapeutics: Research Funding; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma: Research Funding; Pfizer: Research Funding; Agios Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals: Research Funding; CTI Biopharma: Research Funding; Celgene: Other: Grant funding, Research Funding. Huntington:Bayer: Consultancy, Honoraria; Pharmacyclics: Honoraria; DTRM Biopharm: Research Funding; AbbVie: Consultancy; Celgene: Consultancy, Research Funding; Genentech: Consultancy. Gore:Celgene Corporation: Consultancy, Research Funding. Davidoff:Celgene Corporation: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal