Background: The RRMM treatment landscape has increased in complexity in recent years due to the availability of novel agents. In this study, we evaluated treatment patterns over 3 years in real-world RRMM pts treated with regimens containing one or more of the following agents: carfilzomib (K), bortezomib (V), lenalidomide (R), pomalidomide (P), ixazomib (I), daratumumab (D), and elotuzumab (E).
Methods: We retrospectively analysed data from a German longitudinal database (TherapyMonitor) for pts receiving RRMM treatment (2nd-line [2L] and beyond) between January 2016 and December 2018. Patient demographics, treatment details and clinical characteristics were described by line of treatment and regimen for a patients most recent treatment. Treatment patterns were described by year of treatment initiation (2016, 2017 or 2018).
Results: Of the total study population of 2033 RRMM pts, the most recent treatment line was 2L for 1047 and 3L+ for 986 pts. 2L/3L+ pts had a median age of 70/71 years (22%/27% >75 years; most were aged 66-75 years); 57%/61% had ISS stage III; 43%/50% had ECOG ≥2; 10%/9% had renal dysfunction; 56%/56% had ≥1 comorbidity and 14%/17% had received stem cell transplantation at 1L, respectively. In 2L pts, across 2016-2018, R non-triplet (24%) was the most common treatment regimen, with 18% and 10% of pts receiving K+R and D+R triplets, respectively. In 3L+ pts, the most common treatment regimens were D-based (29% [12% non-triplet, 9% D+V triplet and 8% D+R triplet]), P non-triplet (9%), K non-triplet or I/R triplet (both 8%), and R non-triplet (7%). In 2L pts, the most frequent prior treatments were V triplets/non-triplets (45%/19%) and R non-triplets (15%). In 3L+ pts, the most frequent prior treatments were R non-triplets (27%) and K non-triplets (16%). Triplet regimens were mostly administered in 2L to pts ≤65 years. Age had no clear impact on 3L+ treatment patterns. ECOG status impacted treatment patterns in 2L (doublet therapy more frequent in ECOG ≥2) but not in 3L+ (most pts had ECOG ≥2 but were frequently treated with E/D/I triplets).
Regarding trends over time (2016 to 2018), considering all treatments, V triplet+ was the most frequently used regimen prior to 2L (37 to 43%), while V-non-triplet use decreased (32% to 19%) and R-based non-triplet use doubled (7% to 16%). In 2L, K-based regimen use increased from 25% to 38%, mainly due to increased use of K non-triplet (10% to 19%). 2L D-based triplet use increased from 0% to 15% and use of R non-triplet halved (55% to 27%). Patients receiving K- or D-based triplet or R non-triplet at 2L predominantly received V triplet regimens at 1L. Most 2L pts receiving non-triplet regimens also received V or R non-triplets at 1L. These trends suggest a shift in use of R non-triplets from 2L to 1L, replacing 1L V non-triplets and resulting in greater use of 2L K non-triplets.
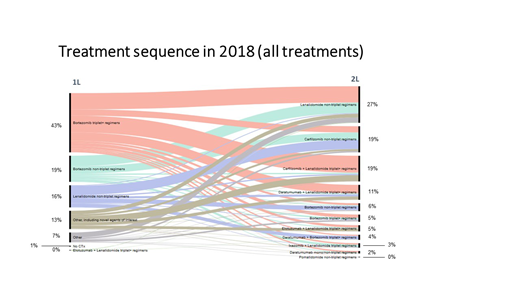
Specifically, for 2L in 2018, of the 27% of pts treated with R non-triplets (Figure), the most common 1L regimens were V-triplet (44%), V non-triplet (29%) and other non-novel agent containing regimens (15%). For the 19% of pts receiving 2L K+R triplet regimen, the most common 1L regimens were V-triplet+ (66%), other regimens containing novel agents (24%) and V and R non-triplets (5% each). Of the 19% of pts receiving 2L K non-triplet regimens, R non-triplet (35%), V non-triplet (28%) and V-triplet+ (24%) were the most common 1L treatments. For the 11% of pts receiving 2L DR-triplet regimens, V-triplet+ (57%), other regimens containing novel agents (20%), R non-triplet (14%), and V non-triplet (6%) were the most common 1L treatments.
Conclusions: Multiple approvals of novel RRMM agents in Europe resulted in changes in the treatment landscape, with a more immediate impact in countries with earlier access to new drugs. In this RRMM population we found increased 1L use of R non-triplets and decreased V non-triplet use between 2016-2018. Use of D-based regimens increased in 2L; P-, I-, and E-based regimens were infrequently used in 2L (≤4% in 2018). Pts receiving 1L non-triplets generally received 2L non-triplet treatment; those receiving 1L triplet generally also received 2L triplet treatment. 3L pts were mostly R-pretreated due to high use of 2L R-based triplets. Germany may serve as an example for the adoption of novel treatments as there is adoption of all RRMM agents approved since 2016.
Merz:Takeda Vertrieb GmbH: Other: Travel grants, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants; Janssen: Other: Travel grants; Abbvie: Other: Travel grants; Celgene: Other: Travel grants. Patel:Amgen: Employment. Kutikova:Amgen: Employment. Lebioda:Amgen: Employment. Schoehl:Amgen: Employment. Kellermann:Takeda: Research Funding; Amgen: Research Funding; BMS: Research Funding; Celgene: Research Funding; Janssen: Research Funding; Sanofi: Research Funding. Goldschmidt:Dietmar-Hopp-Stiftung: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Chugai: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Molecular Partners: Research Funding; MSD: Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundipharma: Research Funding; Janssen: Consultancy, Research Funding; John-Hopkins University: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; John-Hopkins University: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal