Background: Among HLA-well-matched unrelated donor (UD) and umbilical cord blood (CB), HLA-8/8 allele-matched UD transplantation (UDT) showed superior overall survival (OS) to 7/8 allele-matched UDT and CBT, while a similar OS has been demonstrated between the HLA-7/8 allele-matched UDT and the CBT. However, a fair comparison between UDT and CBT is difficult, because graft availability and time required for donor-search is completely different. Once patients relapsed during UD-search period, most of those patients would choose immediate CBT. This means that patients who maintained CR in UD group may have more favorable characteristics, because those patients have been longer in remission and selected as a group of patients who maintained CR. Thus we thought that the factor of "donor-search duration" is important upon conducting a comparative study between UDT and CBT. We planned a clinical study that also taken "donor-search duration" into consideration to compare CBT outcomes with HLA well-matched UDT in a prospective trial setting.
Purpose: The purpose of the current study is to compare the transplant outcomes of HLA-well-matched UDT with those of CBT in a prospective trial.
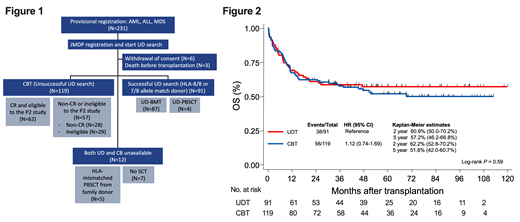
Patients and Methods: From 2007 to 2015, 231 patients were provisionally registered for a single-arm phase 2 study of CBT (manuscript submitted). All provisionally registered patients were subjects of current study (Figure 1). After provisional registration, we attempted to find appropriate UD within a decent time period. After approximately 180 days of donor-search, patients received CBT if an appropriate UD was not available. In total, 91 patients received UDT, and 119 patients received CBT. Six patients withdrew and three died before transplantation. Twelve patients did not receive either UDT or CBT, but five received HLA-mismatched SCT from family donor (seven chose not to receive allogeneic SCT). Of 119 CBT recipients, 62 patients were eligible and registered to a phase 2 clinical trial reported elsewhere. Herein we analyzed transplant outcomes of 91 UDT and 119 CBT (UDT group; 49 AML, 37 ALL, 5 MDS: CBT group; 68 AML, 38 ALL, 13 MDS). Risk factors were analyzed by cox proportional hazard model, and survival estimates were depicted by Kaplan-Meier estimation and tested by log-rank test.
Results: Patient age was median 39 yrs in both UDT and CBT (p=0.80). Patient body weight was median 59kg (37-90kg) in UDT, and median 55kg (35-90kg) in CBT (p=0.10). Sixty-six of 91 (72.5%) in UDT and 114 of 119 (95.8%) in CBT received myeloablative conditioning. More than 90% of patients received Tacrolimus and short-term methotrexate as GVHD prophylaxis in both UDT and CBT. Days from provisional registration to transplant were median 126 days (range, 77-261 days) in UDT, and median 99 days (8-286 days) in CBT (p<0.0001). Diagnosis, disease status at transplant, disease risk index (DRI), and HCT-CI (hematopoietic stem cell transplantation specific comorbidity index) did not differ between UDT and CBT, except disease status at transplant of MDS. First, we assessed risk factors in UDT and CBT separately. In UDT, either DRI or disease status at transplant were significant risk for disease-free survival (DFS) and OS. In CBT, DRI and diagnosis were significant for relapse; DRI was significant for DFS and OS. In multivariate analyses including both UDT and CBT, graft source (UDT vs CBT) was not significant risk for non-relapse mortality (NRM), relapse, DFS, and OS. With adjusted analyses of DFS and OS, UDT and CBT showed similar outcomes: DFS [UDT as reference: CB; Hazard ratio (HR), 1.05 (95% confidence interval (95%CI), 0.70-1.59), p=0.81]. The 2-year DFS was 56.1% (95% CI, 45.0-65.8) for UDT, and 57.5% (95%CI, 47.8-66.1) for CBT (p=0.92). OS [CB; HR, 1.17 (95% CI, 0.78-1.77), p=0.45]. The 2-year OS was 60.9% (95% CI, 50.0-70.2) for UDT and 62.2% (95%CI, 52.8-70.2) for CBT (p=0.59)(Figure 2). With adjusted analyses, relapse and NRM was comparable between UDT and CBT. NRM [CB; HR, 0.75 (95% CI, 0.40-1.42), p=0.38] and relapse [CB; HR, 1.38 (95% CI, 0.79-2.34), p=0.26]. The 2-year relapse rate was 24.9% (95% CI, 16.4-34.3%) for UDT and 29.7% (95%CI, 21.6-38.2%) for CBT (p=0.54). The 2-year NRM was 20.2% (95% CI, 12.6-29.1%) for UDT and 14.7% (95%CI, 8.9-21.7%) for CBT (p=0.40).
Conclusion: Taken donor-search period into consideration, OS after UDT and CBT were similar in a prospective clinical study. CB may be the comparable alternative donor source to UDT.
Terakura:Novartis: Honoraria; Astellas Pharma Inc.: Honoraria; Amgen Astellas BioPharma K.K.: Honoraria; Sumitomo Dainippon Pharma: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Yakult Honsha, Co., Ltd.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria. Nishida:Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Takeda Pharmaceutical Co., Ltd.: Honoraria; MSD K.K.: Consultancy, Honoraria; Amgen Astellas BioPharma K.K.: Honoraria. Sawa:Mundi Pharma: Honoraria; Bristol-Myers Squibb: Honoraria; Sumitomo Dainippon Pharma: Honoraria; Sanofi: Honoraria; Astellas Pharma Inc.: Honoraria; Ono Pharmaceutical Co., Ltd: Honoraria; Otsuka Pharmaceutical: Honoraria; Kyowa-Hakko Kirin: Honoraria; Novartis: Honoraria; Shire: Honoraria; Eisai: Honoraria; Mochida: Honoraria; Pfizer Japan Inc.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Nippon Shinyaku: Honoraria; MSD: Honoraria; Asahi-Kasei: Honoraria; Takeda: Honoraria; Celgene: Honoraria. Miyao:Bristol-Myers Squibb: Honoraria; Celgene Corporation: Honoraria; Novartis: Honoraria. Ozawa:Pfizer Japan Inc.: Honoraria; Kyowa-Hakko Kirin: Honoraria; Astellas Pharma Inc.: Honoraria; Novartis: Honoraria. Goto:Celgene Co., Ltd.: Honoraria; JCR Pharmaceuticals Co., Ltd.: Honoraria; Novartis Pharma Co., Ltd.: Honoraria; Takeda Pharmaceutical Co., Ltd.: Honoraria. Onishi:Sumitomo Dainippon Pharma: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Bristol-Myers Squibb: Honoraria, Research Funding; ONO PHARMACEUTICAL CO., LTD.: Honoraria; Nippon Shinyaku: Honoraria; Kyowa-Hakko Kirin: Honoraria; Pfizer Japan Inc.: Honoraria; Astellas Pharma Inc.: Honoraria; Celgene: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Novartis Pharma: Honoraria; Takeda Pharmaceutical Co., Ltd.: Research Funding; MSD: Honoraria, Research Funding. Fukuhara:Janssen Pharma: Honoraria; Eisai: Honoraria, Research Funding; Chugai Pharmaceutical Co., Ltd.: Honoraria; AbbVie: Research Funding; Celgene Corporation: Honoraria, Research Funding; Zenyaku: Honoraria; Bayer: Research Funding; Nippon Shinkyaku: Honoraria; Kyowa-Hakko Kirin: Honoraria; Mochida: Honoraria; Mundi: Honoraria; Ono Pharmaceutical Co., Ltd.: Honoraria; Takeda Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Gilead: Research Funding; Solasia Pharma: Research Funding. Fujii:Novartis Pharma Co., Ltd.: Honoraria; Kyowa-Hakko Kirin Co., Ltd.: Honoraria. Iida:Chugai Pharmaceutical Co., Ltd.: Research Funding. Endo:Ono: Research Funding. Onizuka:Sumitomo Dainippon Pharma: Research Funding; Astellas: Research Funding; Novartis: Research Funding; pfizer: Research Funding; Chugai Pharma: Research Funding; Bristol-Myers Squibb: Research Funding. Iyama:Otsuka Pharmaceutical Co., Ltd.: Honoraria; Otsuka Pharmaceutical Factory: Honoraria; Astellas Pharma: Honoraria; Daiichi Sankyo: Honoraria; Allexion Pharma: Honoraria; CSL Behring: Honoraria. Nakamae:Japan Blood Products Organization: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceutical: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyowa-Hakko Kirin Co.,Ltd: Honoraria; Nippon Shinyaku: Honoraria; Bristol-Myers Squibb: Honoraria; Shire Japan KK.: Honoraria; Pfizer Japan Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma Inc.: Research Funding; Alexion: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Novartis: Honoraria, Research Funding; Takeda Pharmaceutical Co., Ltd.: Honoraria. Nagata:Janssen Pharmaceutical K.K.: Honoraria; Bristol-Myers Squibb K.K.: Honoraria; Ono Pharmaceutical Co., Ltd: Honoraria; Novartis Pharma K.K.: Honoraria; Celgene K.K.: Honoraria; Takeda Pharmaceutical Co., Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kurahashi:Novartis Pharma Co., Ltd.: Honoraria; Bristol-Myers Squibb, Ltd.: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Takeda Pharmaceutical Co., Ltd: Honoraria. Suzuki:Celgene: Honoraria; Eisai: Honoraria; Novartis: Honoraria; Bristol-Myers Squibb: Honoraria; Kyowa Hakko Kirin: Honoraria; Chugai Pharmaceutical Co.,Ltd.: Honoraria; Meiji Seika: Honoraria; Merck Sharp & Dohme: Honoraria; Takeda Pharmaceutical Co., Ltd.: Honoraria; ONO Pharmaceutical Co., Ltd.: Honoraria; Janssen: Honoraria; AbbVie: Honoraria. Atsuta:Mochida Pharmaceutical Co. Ltd: Honoraria; Kyowa Kirin Co., Ltd: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Janssen Paharmaceutical K.K.: Honoraria. Miyamura:Bristol-Myers Squibb: Honoraria; Novartis: Honoraria; Pfizer Japan Inc.: Honoraria; Otsuka Pharmaceutical: Honoraria; Takeda Pharmaceutical: Honoraria; Kyowa-Hakko Kirin Co., Ltd: Honoraria; CHUGAI PHARMACEUTICAL CO., LTD.: Honoraria; Astellas Pharma Inc.: Honoraria; Celgene: Honoraria. Murata:Bristol-Myers Squibb, Ltd.: Honoraria; Kyowa-Hakko Kirin Co., Ltd.: Honoraria; Celgene Co., Ltd.: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Consultancy, Honoraria; GSK Co., Ltd.: Consultancy; Astellas Pharma Inc.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; JCR Pharmaceuticals Co., Ltd.: Honoraria; Novartis Pharma Co., Ltd.: Honoraria; MSD Co., Ltd.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal