Previous data indicate that pre-transplant flow cytometry measurable residual disease (MRD) in acute myeloid leukaemia (AML) patients in complete remission predicts relapse and survival. Diagnostic laboratories have started to report flow MRD (fMRD) at each AML disease assessment.
We evaluated 83 consecutive patients with AML receiving allogeneic stem cell transplants at King's College Hospital (London, UK) in 2017-2018 to assess the significance of fMRD pre- and post-transplant. Flow MRD detection was based on leukaemia associated immunophenotype and 'difference from normal' strategies, reported at a sensitivity of 0.1%.
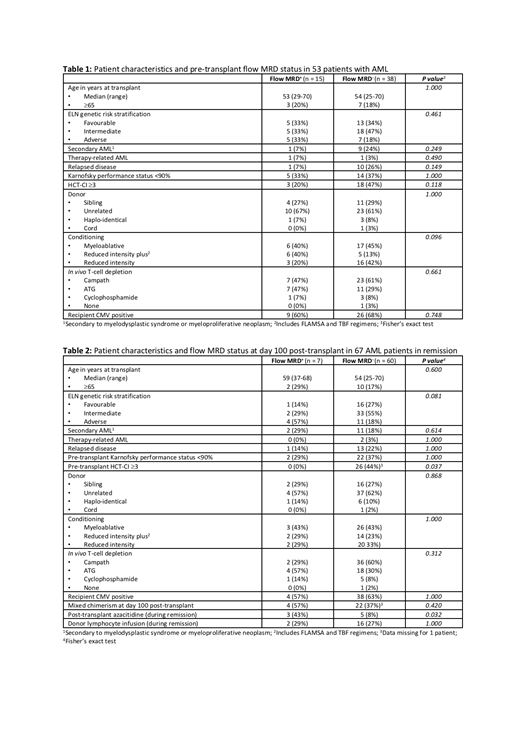
Two timepoints were assessed: pre-transplant (post-induction) and day 100 post-transplant. Pre-transplant, 30 patients were excluded (9 active disease, 21 unknown fMRD) - of the remaining 53 patients in remission (<5% blasts by morphology), 15 were fMRD+ and 38 fMRD-. At day 100 post-transplant, 16 patients were excluded (14 died/relapsed, 2 no fMRD results) - of the remaining 67 patients, 7 were fMRD+ and 60 fMRD- at last assessment. Median follow up was 394 days (range 21-825) from transplant.
Patient characteristics at each timepoint are listed in Tables 1 and 2. Outcome measures were overall survival (OS), relapse free survival (RFS), relapse and non-relapse mortality (NRM). Multivariate Cox regression analysis was performed using the parameters listed in the tables.
Pre-transplant, the only predictor of OS was recipient CMV positivity - HR 0.07 (95% CI 0.01-0.79), p=0.03. RFS was predicted by age ≥65 - HR 0.18 (95% CI 0.05-0.65), p=0.01, secondary AML (sAML) - HR 0.19 (95% CI 0.05-0.77), p=0.02 and CMV positivity - HR 0.16 (95% CI 0.04-0.69), p=0.01. Relapse was predicted by age ≥65 - HR 10.98 (95% CI 1.68-71.73), p=0.01, genetic risk: favourable versus adverse - HR 0.02 (95% CI 0.001-0.04), p<0.01, sAML - HR 14.53 (95% CI 1.26-167.13), p=0.03, performance status <90% - HR 13.35 (95% CI 1.39-128.59), p=0.03, CMV positivity - HR 9.30 (95% CI 1.00-86.38), p=0.05 and fMRD+ - HR 4.75 (95% CI 1.25-18.08), p=0.02. No parameter predicted NRM.
A separate pre-transplant analysis was performed to include molecular MRD (mMRD). Fifty three patients were excluded (9 active disease, 50 not assessed for mMRD, 1 no available result) - in the remaining 23 patients (21 NPM1 mutated, 2 KMT2A rearranged), mMRD was not predictive of any outcome. Eight patients remained mMRD+ in remission post-transplant; 3 were initially mMRD- post-transplant, but became mMRD+ later. Only 2/11 patients relapsed - the others responded to augmentation of the graft versus leukaemia (GVL) effect by reduction of immunosuppression or donor lymphocyte infusion (DLI).
At day 100 post-transplant, OS was predicted by age ≥65 - HR 0.001 (95% CI 2×10-6-0.23), p=0.01 and sAML - HR 0.002 (95% CI 3.6×10-5-0.11), p<0.01. RFS was predicted by CMV positivity - HR 0.07 (95% CI 0.01-0.34), p<0.01, fMRD+ - HR 0.04 (95% CI 0.01-0.36), p<0.01 and DLI - HR 8.93 (95% CI 1.23-64.54), p=0.03. Relapse was predicted by therapy-related AML - HR 10744.98 (95% CI 1.63-71043020.5), p=0.04, CMV positivity - HR 2297.13 (95% CI 6.38-826805.30), p=0.01, fMRD+ - HR 5475.48 (95% CI 27.04-1108709.83), p<0.01 and DLI - HR 0.02 (95% CI 0.002-0.30), p<0.01. No parameter predicted NRM.
In our cohort, recipient CMV positivity was strongly associated with poor outcomes. Age, genetic risk and secondary disease were also important. Pre-transplant fMRD was predictive of relapse but not survival. At day 100 post-transplant, fMRD predicted both relapse and RFS; DLI was associated with a protective effect.
Median time to relapse from fMRD+ at day 100 post-transplant was 89 days - relapse occurred in 5/7 patients. This contrasts with the sensitivity of NPM1 mMRD+ disease to the GVL effect. Further work is required both to confirm our findings in a larger cohort and to determine how best to manage patients who are fMRD+ post-transplant.
Off Label Use: Azacitidine (Vidaza) - used for prevention of relapse after allogeneic stem cell transplantation for acute myeloid leukaemia. de Lavallade:Bristol Myers Squibb: Research Funding. Mufti:Celgene: Consultancy, Research Funding.
Azacitidine (Vidaza) - used for prevention of relapse after allogeneic stem cell transplantation for acute myeloid leukaemia
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal