Older patients with advanced hematologic malignancies are increasingly considered for allogeneic hematopoietic cell transplantation (allo-HCT). However, survival outcomes, especially non-relapse mortality (NRM) in these patients remain suboptimal due to multi-morbidity and geriatric vulnerabilities. We and others have shown previously that pre-transplant multi-morbidity, and functional limitation and post-HCT geriatric syndromes of delirium and fall significantly impact allo-HCT outcomes. Sarcopenia, an accelerated loss of muscle mass and function, has been increasingly recognized to affect HCT outcomes. However, it remains unknown whether sarcopenia is a simple surrogate of multi-morbidity or functional impairment and whether post-transplant sarcopenia also impacts outcomes.
From our institutional database and the electronic medical record, we identified 146 lymphoma patients 50 years or older who were transplanted at our institution from 2008 to 2018 using reduced-intensity/nonmyeloablative conditioning and a matched related or unrelated, mismatched unrelated, or haploidentical donor, and who had either a PETCT or CT within 60 days of HCT. Two adjacent axial images within the same series at the third lumbar vertebra were selected for the analysis of total muscle cross-sectional area (cm2) and of total fat (subcutaneous and visceral adipose tissues) cross-sectional area (cm2) using MIM software. Skeletal muscle cross-sectional area was normalized for stature and reported as skeletal muscle index in cm2/m2. Sarcopenia was defined as a skeletal muscle index <41 in women, <43 in men with BMI <25, and <53 in men with BMI ≥25. Baseline characteristics, HCT outcomes, and geriatric assessment domains were obtained as previously described.
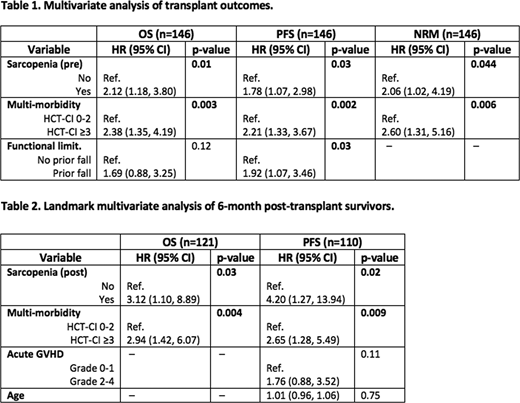
Before allo-HCT, 80 (55%) patients were sarcopenic. Baseline demographic, transplant, and geriatric characteristics were well matched among the two groups except for slightly more females in the sarcopenic group (38% versus 21%, p=0.046). In multivariable analysis (Table 1), pre-HCT sarcopenia was significantly associated with overall survival (OS) with hazard ratio (HR) of 2.12 (95% CI 1.18 - 3.80, p=0.01); progression-free survival (HR 1.78, 95% CI 1.07 - 2.98, p=0.03); and NRM (HR 2.06, 95% CI 1.02 - 4.19, p=0.044). Multi-morbidity (HCT-CI ≥3) was independently associated with OS, PFS, and NRM, while functional impairment as measured by prior fall was significantly associated with PFS (Table 1).
One hundred twenty-eight patients had CT images within 3-6 months post-HCT. Comparing to pre-transplant values, there were median decrease in total lean body mass of -2.86 kg (range -16.68 -9.87, p<0.001), and median decrease in total body fat mass of -2.23 kg (range -14.2 -5.67, p<0.001). Seventy percent of patients were sarcopenic post-transplant. We performed 6-month landmark analysis of OS, PFS, and NRM (Table 2). Post-HCT sarcopenia was significantly associated with OS (HR 3.12, 95% CI 1.10 - 8.89, p=0.03) and PFS (HR 4.2, 95% CI 1.27 - 13.94, p=0.02), but not NRM. Multi-morbidity (HCT-CI ≥3) was again independently associated with OS, PFS, and NRM in the landmark analyses, while grade 2-4 acute GVHD was significantly associated with PFS only in univariate analysis (Table 2).
While limited by the retrospective design, single diagnosis of lymphoma, and inability to establish causal relationships among GVHD, steroid use, and post-HCT sarcopenia, our findings nevertheless illustrated the high prevalence of sarcopenia in older allo-HCT recipients. Importantly, we demonstrated significant negative impact of both pre- and post-HCT sarcopenia on survival which were independent of geriatric multi-morbidity, functional impairment, and acute GVHD. While requiring prospective confirmation, the temporal pattern and adverse survival impact of sarcopenia warrants preemptive, targeted, longitudinal, and multidisciplinary interventions to improve HCT outcomes for older patients.
Sauter:Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy; GSK: Consultancy; Genmab: Consultancy; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy; Celgene: Consultancy; Sanofi-Genzyme: Consultancy, Research Funding; Juno Therapeutics: Consultancy, Research Funding. Perales:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; NexImmune: Membership on an entity's Board of Directors or advisory committees; Medigene: Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MolMed: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Kyte/Gilead: Research Funding; Miltenyi: Research Funding. Scordo:Angiocrine Bioscience, Inc.: Consultancy; McKinsey & Company: Consultancy. Giralt:Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy; Actinium: Consultancy, Research Funding; Johnson & Johnson: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Kite: Consultancy; Jazz Pharmaceuticals: Consultancy; Amgen: Consultancy, Research Funding; Miltenyi: Research Funding; Takeda: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal