Introduction:
Daratumumab is a human immunoglobulin (IgG-κ) that targets CD38 abundantly expressed on plasma cells. Data is emerging to show its efficacy as part of induction therapy for newly diagnosed multiple myeloma (MM) patients. Hematopoietic stem cells have been shown to express CD38 on the cell surface and therefore during mobilization, there is a theoretical risk of circulating daratumumab binding to and having downstream effects on these cells.
Methods:
We conducted a retrospective review of MM patients treated with daratumumab prior to stem cell collection and autologous stem cell transplant (ASCT) to identify any effects daratumumab therapy may have on the efficacy of the stem cells in bone marrow recovery. The study was conducted at Mayo Clinic Rochester from February 2018 to May 2019. Granulocyte colony-stimulating factor was the preferred agent used for stem cell mobilization and plerixafor was added in a demand-adapted fashion. All patients received melphalan infused at day -1 before their ASCT and the decision about dosing (200mg/m2 vs 140mg/m2) was at the physicians' discretion. Neutrophil engraftment was defined as the first date of three consecutive neutrophil counts >0.5 x 10^9/L and platelet engraftment was defined as the first date of three consecutive platelet counts >50 x 10^9/L in the absence of platelet transfusion in the preceding 7 days.
Results:
We identified 12 patients who received daratumumab as part of their first induction regimen (daratumumab cohort) and compared them to 129 patients who did not receive daratumumab prior to stem cell mobilization and transplant during the study period (no daratumumab cohort). Of the daratumumab cohort, 11 patients received daratumumab, ixazomib, lenalidomide, and dexamethasone and one received daratumumab, cyclophosphamide, bortezomib and dexamethasone. No differences were noted in terms of melphalan dose or the number of CD34+ stem cell infused. The median time from the last dose of daratumumab to stem cell collection was 3.9 weeks.
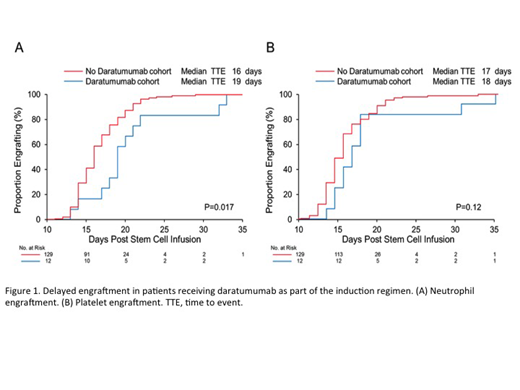
In patients receiving daratumumab, the median time for neutrophil engraftment was three days longer compared with those who did not receive daratumumab (median of 19 days vs. 16 days, P=0.017) (Figure 1, A). Median platelet engraftment was delayed by one day, although this was not statistically significant (median of 18 days vs. 17 days, p=0.12) (Figure 1, B). A total of 6 patients (50%) from the daratumumab cohort and 52 (40%) from the no daratumumab cohort required hospital admission. Of these admissions, there was no difference between the two groups in rates of admission for fever (50% in the daratumumab group vs. 42% in the no daratumumab group, P=0.7).
Conclusion:
Our case series provides the first report of daratumumab delaying engraftment post transplant in myeloma patients receiving it prior to stem cell collection. A better characterization of this phenomenon is important given the increasing use of daratumumab as front line therapy prior to ASCT in patients with myeloma.
Gertz:Prothena: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Spectrum: Honoraria, Research Funding; Alnylam: Honoraria; Ionis: Honoraria. Lacy:Celgene: Research Funding. Kapoor:Sanofi: Consultancy, Research Funding; Glaxo Smith Kline: Research Funding; Cellectar: Consultancy; Janssen: Research Funding; Amgen: Research Funding; Takeda: Honoraria, Research Funding; Celgene: Honoraria. Dispenzieri:Alnylam: Research Funding; Celgene: Research Funding; Takeda: Research Funding; Pfizer: Research Funding; Janssen: Consultancy; Intellia: Consultancy; Akcea: Consultancy. Dingli:alexion: Consultancy; Janssen: Consultancy; Millenium: Consultancy; Rigel: Consultancy; Karyopharm: Research Funding. Kumar:Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal