Background: In recipients of allogeneic hematopoietic stem cell transplantation (HSCT), organ toxicity is a barrier to administering high-intensity conditioning regimens. We hypothesized that determinants of acute organ toxicity are specific to individual conditioning regimens. We sought to characterize toxicities across common transplantation regimens, evaluate their prognostic implication, and derive predictors of severe toxicity at the regimen level.
Methods: This retrospective study included adults undergoing first allogeneic HSCT at a single center between the years of 2001 and 2014 (median: 2010). Patients received grafts from matched sibling or unrelated donors and were conditioned with any of the following regimens: Cyclophosphamide + TBI (Cy/TBI), Busulfan + Cyclophosphamide (Bu/Cy), Fludarabine + 12.8 mg Busulfan (Flu/Bu4), Fludarabine + 6.4 mg Busulfan (Flu/Bu2), Fludarabine + 36-42 gr/m2 Treosulfan (Flu/Treo), and Fludarabine + 100-140 mg/m2 Melphalan (Flu/Mel). Toxicities were defined by the KDIGO scale for acute kidney injury (AKI) and by the CTCAE v. 5.0 for increases in total bilirubin, AST, ALT, and alkaline phosphatase (Alk. Phos.) The incidence of toxicities from the start of conditioning through 30 days post-transplantation was tabulated by regimen. Risk factors for severe organ toxicity were assessed within each regimen cohort using multivariable logistic regressions.
Results: In a cohort of 707 patients, the median age was 52 years. The main indications for transplantation were acute leukemia (57%), myelodysplastic syndrome (13%), and aggressive lymphoma (9%). Graft-versus-host-disease prophylaxis included methotrexate in 80% of patients, and 56% received anti-thymocyte globulin (ATG). The most common regimens were Flu/Treo (n = 160) and Bu/Cy (n = 141). As expected, patient characteristics varied between regimens.
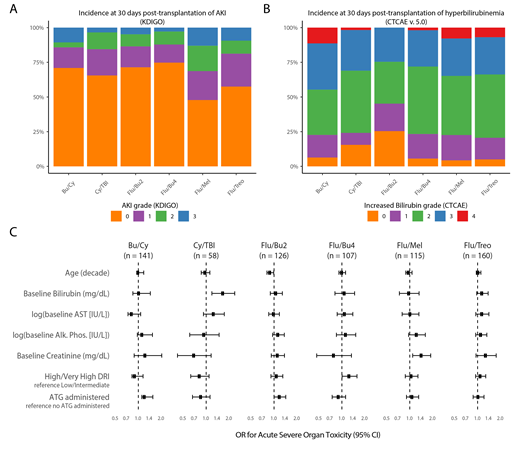
The incidence of AKI and increased serum bilirubin in each regimen is shown in Figure 1A and 1B, respectively. Sinusoidal-obstructive syndrome (6% overall) accounted for only 17% of gr. ≥ 3 bilirubinemia in the entire cohort. Elevations in AST, ALT, and Alk. Phos of gr. ≥ 3 were not common (<8%). In multivariable logistic regression, AKI gr. ≥ 2, increased bilirubin gr. ≥ 3, AST gr. ≥ 3, and Alk. Phos. gr. ≥ 2 were associated with increased 100-day mortality (p < 0.05). Acute severe organ toxicity (ASOT) was defined as the occurrence of any of these toxicities. ASOT had an odds ratio (OR) of 3.4 (95% CI: 2.2-5.3) for 100-day mortality. Within each regimen, we studied the relationship between ASOT and transplantation/patient characteristics (Figure 1C). Elevations in baseline bilirubin were predictive of ASOT in Cy/TBI (OR: 1.68 [1.19-2.37]), while increasing creatinine was predictive in patients conditioned with Flu/Mel (OR: 1.43 [1.09-1.88]). High-risk disease (DRI) was associated with increased risk in patients receiving Flu/Bu4 (1.26 [1.01-1.58]). In patients treated with Bu/Cy, administration of ATG increased the risk of ASOT (1.31 [1.11-1.55]).
Conclusion: Allogeneic stem cell transplantation recipients are at high risk for acute organ damage. We describe patterns of renal and liver toxicity across several regimens. Determinants of acute severe organ toxicity, defined as those associated with short-term mortality, are regimen dependent. Our findings suggest that these factors should be considered when selecting the preparative regimen. While requiring validation, the newly-defined composite endpoint of acute severe organ toxicity (ASOT) may be valuable in studying transplantation strategies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal