Background: Allogeneic hematopoietic cell transplantation (alloHCT) is a complex therapy which can induce a multi-factorial cascade of complications, and potentially lead to patient death. The triggering event(s), sequence and severity of such complications can significantly differ between patients, but in many cases, a so-called "multi-organ failure" (MOF) is usually reported as the leading cause of death. However, a patient's clinical course can be very heterogeneous across and within cause-specific mortalities. Moreover, comorbidities present prior to alloHCT carry their own risks and represent additional confounding factors. Therefore, identification of the exact initial trigger or event leading to MOF in alloHCT patients is a critical step towards early intervention and improvement of patients' outcome. The goal of the current study was to establish and identify the exact cause of death in alloHCT patients where MOF was considered to be the main cause of death. Of note, we specifically focused on VOD/SOS because this life-threatening complication has a mortality exceeding 80% in severe cases, ending usually in MOF, and because VOD/SOS has subtle and dynamic evolution features which are not easy to capture, but could be potentially controlled by appropriate therapy (eg. defibrotide).
Patients and Methods: For the purpose of this analysis, we randomly identified 241 adult patients (42% female; median age: 50 years; range 19-73) with acute leukemia (72% AML, 25% ALL, 3% other) allografted between 2010 and 2018 from a matched sibling (29%), unrelated (61%) or haploidentical donor (10%). All patients were reported to the EBMT registry to have died from MOF. Karnofsky score at time of alloHCT was >90 in 87% of patients. Seventy-three percent of patients underwent transplant in complete remission, and conditioning was myeloablative in 70%. Sixty patients (25%) received VOD/SOS prophylaxis treatment, mainly consisting of ursodiol and/or heparin. Patients' files were reviewed in detail in order to capture all early signs and symptoms which occurred prior to MOF, based on the classical Baltimore criteria, modified Seattle criteria, and/or the newly published EBMT criteria. These criteria included bilirubin levels, the presence of hepatomegaly or painful hepatomegaly, ascites, percentage weight gain, hemodynamic instability, and ultrasound/histologically proven VOD/SOS.
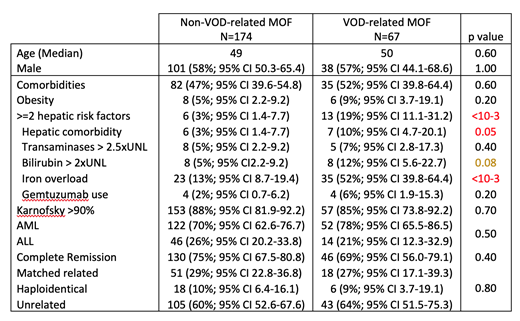
Results: Using one or more of the above criteria defining VOD/SOS, we identified a total of 67 (28%) patients for whom VOD/SOS could be considered as the trigger for MOF and the leading cause of death. Interestingly, among these 67 patients, only 22 (33%) were originally reported by the centers as having developed VOD/SOS leading to MOF post-transplant. When comparing the group of 67 patients dying of VOD/SOS-related MOF and the remaining 174 patients dying of MOF not related to VOD/SOS (please see attached table), a multivariate regression analysis identified a significant increase in VOD/SOS incidence (odds ratio 3.9; 95%CI, 2.42-6.33; p<10-3) with the presence of hepatic risk factors (scoring from 0 to 5): history of liver disease, transaminases 2.5 times and bilirubin 2 times above normal limits at time of alloHCT, the presence of iron overload and the use of gemtuzumab. Of note, there was no significant detrimental impact of the use of total body irradiation or disease status. The median survival for patients with VOD/SOS-related MOF was 47 days (95%CI, 34-84) compared to 175 (95%CI, 121-234) days for patients with non-VOD/SOS related MOF.
Conclusion: The above results suggest that VOD/SOS-related MOF is an under-reported cause of death. It is actually a relatively frequent cause of MOF, accounting for at least 28% of deaths that would otherwise been attributed to MOF of unknown origin. While the MOF concept is a widely used cause of death after alloHCT, having precise categories of cause-specific death from MOF should allow (whenever possible), for earlier specific therapeutic intervention (eg. defibrotide), thus contributing to improved patient outcome.
Labopin:Jazz Pharmaceuticals: Honoraria. Schaap:Celgene: Consultancy; Novartis: Consultancy. Snowden:Jazz: Honoraria; IDMC: Honoraria; Mallinckrodt: Honoraria; Kiadis: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; Janssen: Honoraria. Mohty:Jazz Pharmaceuticals: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal