Background:
CAR T therapy is FDA approved for specific relapsed/ refractory (R/R) B cell lymphomas and acute lymphoblastic leukemia (ALL), and clinical trials are ongoing for R/R multiple myeloma (MM). Cytopenias have been observed post-CAR T, yet there is minimal data delineating the pathobiology and trends. We report the largest series to our knowledge thus far, of hematological recovery and factors affecting count recovery after CAR T.
Methods:
We retrospectively reviewed adult patients who received CAR T for R/R B cell lymphomas after FDA approval and those treated for R/R B cell ALL (NCT01044069) and MM (NCT03070327) at our center. Blood counts were collected for up to 12 months or until censored for relapse, progression or initiation of chemotherapy/ conditioning for autologous or allogeneic stem cell transplantation (HCT)/ subsequent treatment with CAR T. Only patients with follow-up > 30 days were included. "Recovery" for the respective blood count was defined as hemoglobin > 8g/dL and platelets > 50,000/µL without transfusion support in 2 weeks and 1 week, respectively; absolute neutrophil count (ANC) > 1,000/µL and white cell count (WBC) > 3,000/µL without growth factor support in 2 weeks. "Normalization" was defined as normal range for the laboratory; hemoglobin > 11.2g/dL in women and 12.5g/dL in men, platelets > 160,000/µL, ANC > 1,500/µL and WBC > 3,000/µL without transfusion support as above. "Complete count recovery" refers to recovery per above criteria in all 4 counts. Categorical variables were compared using Fisher's exact test and continuous variables using the Wilcoxon rank-sum test.
Results:
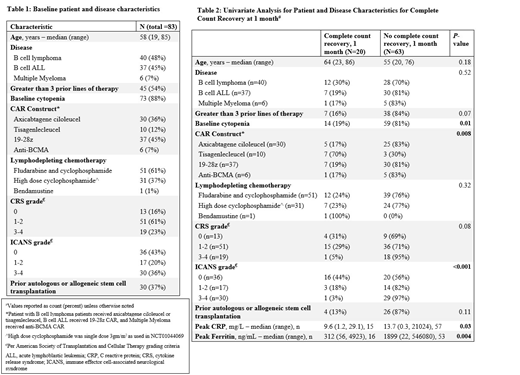
Eighty three patients were included (Table 1). Using the noted nadir values, grade 1-2 and 3-4 anemia was seen in 22% and 78%, thrombocytopenia in 29% and 66%, neutropenia in 3% and 96% while leucopenia in 0% and 100%, respectively. During the follow-up, 66% patients received packed red cell transfusion, 52% received platelet transfusion and 62% received growth factor support.
By 1 month (n=83), recovery of hemoglobin, platelets, ANC and WBC was noted in 61%, 51%, 33% and 28%, respectively. At 3 months (n=41), these respective percentages were 93%, 90%, 81% and 59%. All patients had recovered hemoglobin and platelet count by 4 months (n=17), and ANC by 9 months (n=14). By 3 months, normalization of hemoglobin, platelets, ANC and WBC was noted in 39%, 34%, 71%, and 39%, respectively.
Upon examination of potential variables in a univariate model, lack of recovery of hemoglobin, platelets, ANC and complete counts recovery at 1 month was statistically significantly associated with type of CAR construct, higher grade (grade 3-4 vs grade 1-2 vs none) cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) as well as a higher peak CRP and ferritin (data for complete count recovery in Table 2). Additionally, lack of hemoglobin recovery at 1 month was associated with lymphodepletion using high dose cyclophosphamide (recovered vs no recovery in 58% vs 42%, p=0.01) and diagnosis of ALL (65% vs 35%, p = <0.001). Similarly, inability to recover platelets at 1 month was significantly associated with prior HCT (63% vs 36%, p =0.04); while ANC was associated with prior HCT (87% vs 13%, p = 0.004) and > 3 prior lines of therapy (78% vs 22%, p = 0.04). At 3 months, absence of complete count recovery was associated only with the CAR T construct utilized. A multivariate logistic regression model resulted in wide confidence intervals due to small size of subgroups, hence leading to unreliable point estimates (data not shown).
Conclusions:
Our study shows that blood counts recover in most patients who have not progressed or received additional therapy by 3 months post-CAR T. The association of count recovery with severity of CRS, ICANS as well as inflammatory marker levels indicates that inflammatory response post-CAR T influences hematological recovery in these patients. The association of count recovery and CAR construct can be influenced by underlying diagnosis as specific CAR constructs were used for specific diagnosis. Since patients with no disease response were excluded and were censored at progression, these effects are less likely to be affected by disease response; however the association of depth of response could not be evaluated in this study. These results warrant future studies to understand underlying mechanisms of inadequate recovery.
Scordo:McKinsey & Company: Consultancy; Angiocrine Bioscience, Inc.: Consultancy. Sauter:Kite/Gilead: Consultancy; Precision Biosciences: Consultancy; Genmab: Consultancy; Celgene: Consultancy; GSK: Consultancy; Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy. Santomasso:Novartis: Consultancy; Kite/Gilead: Consultancy; Juno/Celgene: Consultancy. Palomba:Noble Insights: Consultancy; Hemedicus: Other: Immediate Family Member, Speakers Bureau ; Evelo: Other: Immediate family member, Equity Ownership; MSK (IP for Juno and Seres): Other: Immediate Family Member, Patents & Royalties - describe: intellectual property rights ; Kite Pharmaceuticals: Other: Immediate Family Member, Membership on an entity's Board of Directors or advisory committees; Merck & Co Inc.: Other: Immediate Family Member, Consultancy (includes expert testimony); Seres Therapeutics: Other: Immediate Family Member, Equity Ownership and Membership on an entity's Board of Directors or advisory committees; STRAXIMM: Other: Immediate Family Member, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees. Shah:Amgen: Research Funding; Janssen: Research Funding. Batlevi:Juno Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Smith:Celgene: Consultancy, Patents & Royalties, Research Funding; Fate Therapeutics and Precision Biosciences: Consultancy. Giralt:Celgene: Consultancy, Research Funding; Takeda: Consultancy; Sanofi: Consultancy, Research Funding; Amgen: Consultancy, Research Funding. Brentjens:Celgene: Consultancy; JUNO Therapeutics: Consultancy, Patents & Royalties, Research Funding. Park:Takeda: Consultancy; Novartis: Consultancy; Kite Pharma: Consultancy; Incyte: Consultancy; Allogene: Consultancy; Amgen: Consultancy; AstraZeneca: Consultancy; Autolus: Consultancy; GSK: Consultancy. Perales:Bristol-Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; Medigene: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Kyte/Gilead: Research Funding; Miltenyi: Research Funding; MolMed: Membership on an entity's Board of Directors or advisory committees; NexImmune: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees. Mailankody:Celgene: Research Funding; Juno: Research Funding; Janssen: Research Funding; Takeda Oncology: Research Funding; CME activity by Physician Education Resource: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal