Introduction:Acute graft-versus-host disease (aGVHD) can develop into a life-threatening complication after allogeneic hematopoietic cell transplantation (HSCT). The gastrointestinal (GI) tract is considered to play a key role in the pathophysiology of aGVHD, where the disease-process can start and be one of the target organs.The clinical presentation of acute aGVHD in the lower GI tract includes secretory diarrheas, sometimes bloody stools, abdominal pain with or without paralytic ileus. In the upper GI, symptoms such as nausea, anorexia, vomiting and weight loss are common. First-line therapy for aGVHD includes steroids at doses of 1-2 mg/kg /day. Approximately 50-60 % of the patients with aGI-GVHD fail to respond to steroids and their outcomes are very poor with a long-term mortality between 70-90 %.
Bone marrow derived mesenchymal stromal cells (BM-MSCs) were introduced as a novel cell-based therapy by us for aGVHD a few decades ago, but not all patients responded, and many patients died due to invasive fungal infection. Decidua stromal cells (DSCs), isolated from the fetal membrane, express a stronger immunosuppression activity compared to other sources of MSCs and have been used to treat patients with severe steroid-refractory GVHD (SR-GVHD). The placenta protects the haploidentical fetus from the mother's immune system during pregnancy. So far, no severe side effects have been seen using DSCs.
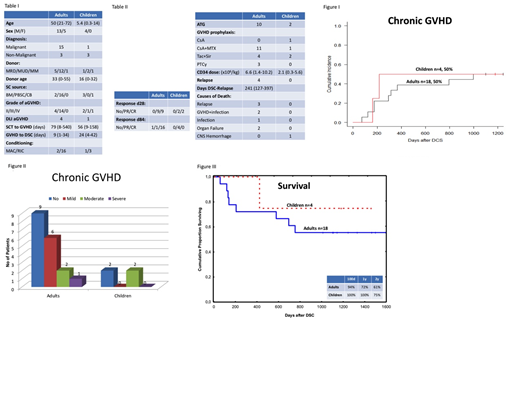
Material and Methods;DSCs were isolated from full-term placentas following elective Caesarian-sections using a GMP facility. The study included 18 adult patients, median age was 50 (21-72) years and 4 pediatric patients, median age of 5.4 (0.3-14). The diagnosis was verified by GI biopsy. Histology confirmed GVHD, grade II-IV (Table 1). The study period took place between 2012 and 2016. Four adults presented with GVHD grade II and fourteen had GVHD grade III. In the pediatric population two patients were diagnosed with GVHD grade II, one child with GVHD grade III and one with GVHD grade IV.Twelve patients were steroid refractory after > 7 days and ten patients after>3 days. Subsequently DSCs were given at a median dose of 1.2 (0.9-2.9) x 106cells/kg and the frequency of 2 (1-6) doses/patient, given one week apart. Viability of thawed DSCs was 95% (89-100) and median passage number of infused cells was 4 (2-4). The study was approved by the regional ethical committee.
Results:In summary all patients responded to the DSCs. Complete resolution of GVHD was seen in16 patients from the adult population and 2 showed partial response at day 28. All children presented a partial response at day 28 (Table II). The cumulative incidence of chronic GVHD was observed in 50% including both the adult and pediatric population, in accordance to the NIH GVHD severity scoring (Fig 1 and 2). Nine patients died, 3 from relapse, 1 acute GVHD in combination with septicemia, 1 zygomycetes infection, 1 liver insufficiency, 1 multiorgan failure, 1 chronic GVHD with obstructive bronchiolitis and one child died from cerebral hemorrhage (Table II). Four years transplant related mortality was 28.6% and overall four years survival was 57% and 75 % in the adult and pediatric group respectively (Fig 3).
Conclusion:There is a necessity for an effective therapy, with fewer side effects, when the patient develop SR-GVHD. Overall long time-survival in patients with steroid-refractory GVHD is poor, with a long-term mortality up to 70-90 %. In our study, decidua stromal cells showed a strong immunosuppression with a high efficacy in SR-GVHD patients to achieve long-term survival. To conclude, DSCs seem to be a useful cell therapy for severe acute GVHD. Randomized trials are under way.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal