Background: Treatment of thrombosis relies on prompt resolution of thrombi to restore blood flow to avoid ischemic injury. However, our understanding of the step-by-step process of how thromboses resolve remains limited due in large part to the lack of sufficient technologies. In addition, as thromboses require days to weeks to resolve, existing in vitro and in vivo systems cannot monitor this process, especially in the microvasculature where thrombi are difficult to visualize. As such, questions such as how do the cellular and biochemical composition of a clot change as it resolves and how do hemodynamics affect this process remain unanswered. This is particularly important in thromboinflammatory conditions such as autoimmune/inflammatory disorders in which patients are chronically at risk for microvascular thromboses but the pathophysiology and therefore optimal therapies remain unclear. Thus, a pressing need exists for an assay that assesses how microvascular thromboses resolve over long time scales, especially under thromboinflammatory conditions. We recently developed a microfluidic system that assesses microvascular events, including endothelial dysfunction and permeability, in response to proinflammatory signals over months (Qiu, Nature Biomed Eng. 2018). Here we leverage this system to monitor not only how microvascular thromboses form but, importantly, how they resolve over long timescales. Moreover, our system also enables the monitoring of how antithrombotic drugs and anticoagulants "work" in the context of existing inflammatory thrombi, which will provide insight into the pathophysiology as well as provide evidence for the use of different therapies.
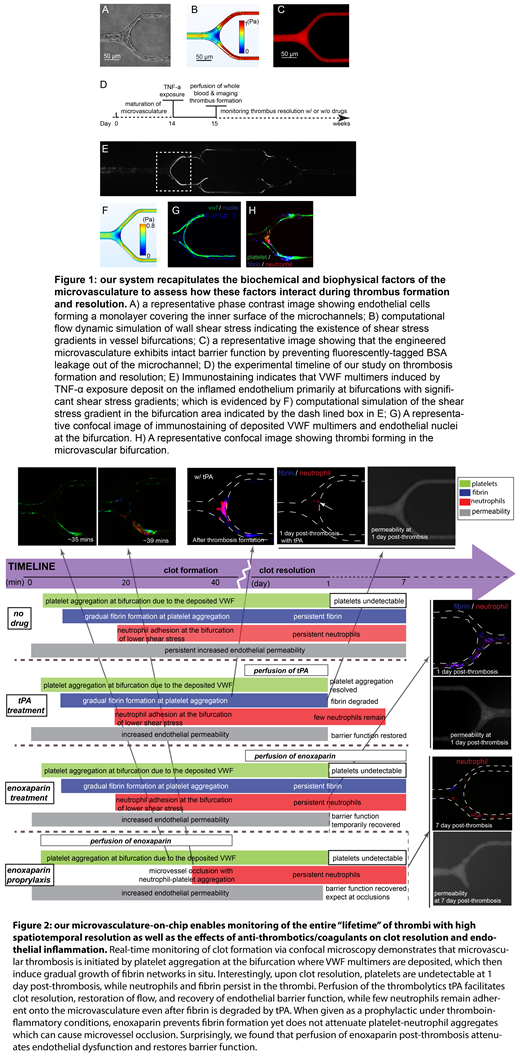
Results and Discussions: Our engineered microvasculature on chip recapitulates the biophysical microenvironment, such as microvessel size, geometry, wall shear stress, and shear gradients (Fig 1A-C), as well as the biological microenvironment, such as perfusion of whole blood, endothelial cells activated with inflammatory mediators, and vascular permeability, to assess how these factors interact during microvascular thrombi formation and resolution over long timescales (Fig 1D). As the entire "lifetime" of a clot is monitored with high spatiotemporal resolution (Fig 2), how the innate immunity, platelets, and coagulation cascade interact during microvascular thrombosis and resolution can be systematically studied. Interestingly, exposure of the microvasculature to TNF-α induces VWF multimers that deposit onto the inflamed endothelium at bifurcations of the smallest vessels, where wall shear stress gradients exist. The deposited VWF multimers then induce platelet aggregation in the bifurcation within minutes and is accompanied with gradual fibrin formation (Fig 1E-H). Neutrophils adhere to the inflamed endothelium at a relatively later stage primarily in areas with lower wall shear stress, aggregating with platelets and incorporating with the growing fibrin mesh. Interestingly, as thrombi start to resolve, platelets are mostly undetectable by 1 day post-thrombosis, while neutrophils and fibrin persist, occluding flow and preventing endothelial barrier function recovery.
With this novel system, we are also able to monitor, for the first time, the effects of commonly used anticoagulants such as enoxaparin on clot resolution (Fig 2).When given as a prophylactic under thromboinflammatory conditions, enoxaparin prevents fibrin formation yet does not attenuate platelet-neutrophil aggregates, highlighting the critical role of interaction of platelets and neutrophils in microvascular occlusion. Surprisingly, enoxaparin also decreases endothelial dysfunction and restores barrier function, suggesting that a significant part of enoxaparin's antithrombotic effects may be endothelial in nature. While post-thrombosis overnight perfusion of the thrombolytic tPA expectedly results in complete fibrin degradation and restoration of flow, tPA also surprising induces endothelial barrier function.
Conclusions: We have, for the first time, developed a perfusable vascularized thrombus resolution assay that enables the tracking of inflammatory thrombi over weeks and is ideal for studying antithrombotic drugs effects and how they may restore microvascular barrier function. Studies assessing the formation of microvascular emboli in this context are ongoing.
Lam:Sanguina, LLC: Equity Ownership; Sanguina, LLC: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal