Background: Myelodysplastic syndrome with ring sideroblasts (MDS-RS) is defined by the World Health Organization (WHO) as ring sideroblasts (RS) ≥15% or ≥5% with associated SF3B1 mutation and no excess blasts (EB). In MDS-RS, SF3B1 mutation defines a homogenous group with isolated erythroid dysplasia and favorable prognosis (Malcovati et al. Blood 2015). Given the separate WHO classification, patients with MDS-EB frequently are not tested for RS. SF3B1-wild type (wt) MDS with RS also has not been well characterized. Therefore, herein we characterized MDS with RS, focusing on SF3B1-wt and implications of molecular subsets.
Patients and Methods: Between 2013 and 2018, 157 MDS and MDS/MPN patients with RS ≥5% and next generation sequencing performed within 6 months of diagnosis at Moffitt Cancer Center were identified with clinical variables obtained at date of diagnosis. Quantification of RS was performed in all cases by hematopathology. Baseline characteristics were compared by Fisher's exact test (categorical variables) and Mann-Whitney test (continuous variables). Survival estimates were calculated using the Kaplan-Meier method from date of diagnosis and groups were compared using log-rank test. Multivariate survival analysis performed by means of Cox proportional hazards regression. Pearson correlation coefficient was used in correlative analyses.
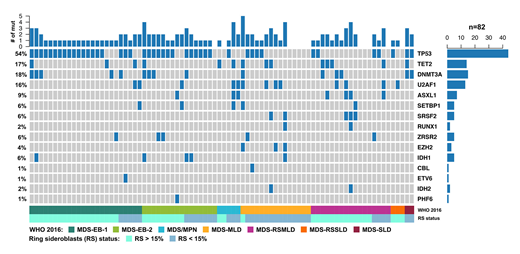
Results: A total of 75 SF3B1-mutant (mt) and 82 SF3B1-wt cases with MDS (141) or MDS/MPN (13) and RS were identified. Median age was 71 years (38-89) with male and Caucasian predominance (62% and 94%, respectively). In the SF3B1-wt cohort, there were 77 MDS and 5 MDS/MPN patients. The MDS patients consisted of 2 MDS-SLD, 15 MDS-MLD, 3 MDS-RS-SLD, 17 MDS-RS-MLD, 24 MDS-EB1 and 16 MDS-EB2. The majority of SF3B1-wt patients (58%) were high or very high risk based on the Revised International Prognostic Scoring system (IPSS-R). Median RS% was significantly lower in SF3B1-wt compared to SF3B1-mt (18% (5-50) vs 35% (5-83) p <0.0001). TP53 was the most common mutation (54%) in the SF3B1-wt cohort (n=44; Figure 1). Additional mutations observed in >10% of the SF3B1-wt cohort were DNMT3A 18% (n=15), TET2 16% (n=13) and U2AF1 16% (n=13). Non-SF3B1 spliceosome mutations represented 27% (n=22) of the SF3B1-wt cohort. TP53-mt and non-SF3B1 spliceosome-mt were observed at significantly higher prevalence in SF3B1 wt vs mt patients (p<0.0001 and p=0.003). In univariate analysis, IPSS-R, TP53 and DNMT3A were associated with worse overall survival in SF3B1-wt patients (OS). In multivariate analysis including age, IPSS-R and BMT, only TP53 was an independent covariate for inferior survival (HR 6.3; 95% CI 2.4-16.6 p<0.0001).
Given the high frequency of mutations, we then focused on TP53-mt RS patients. In the total cohort of patients with RS≥5%, 77% of MDS-EB1 and 68% of MDS-EB-2 were TP53-mt. In SF3B1 wt patients with RS≥5% and excess blasts, TP53 mutation was identified in 79% (n=19) and 81% (n=13) of MDS-EB-1 and MDS-EB-2 patients, respectively (p<0.0001 TP53-mt MDS-EB vs other). 3 patients were co-mutant for TP53 and SF3B1. Increased RS as defined as >15% vs 5-15% resulted in improved OS in the TP53-mt cohort (median OS 13.5 vs 8.6 months; HR 0.36 95% CI 0.14-0.93 p=0.034). In multivariate analysis including age, IPSS-R and BMT, the survival advantage was maintained (HR 0.35 95% CI 0.14-0.93 p=0.034). Increased RS did not significantly improve OS in any other somatic mutation. Response to hypomethylating agents was similar between TP53-mt RS >15% vs 5-15% (Complete remission (CR) 21% vs 17% p=1.0 and overall response rate (ORR) 52% vs 42% p=.72). No TP53-mt RS patients responded to lenalidomide (0/4). The proportion of patients receiving allogeneic stem cell transplant was similar between TP53-mt RS >15% vs 5-15% (14% vs 22% p=0.69). There was no difference in distribution of TP53-mt between 5-15% RS cohort vs >15% (55% n=26 vs 51% n=18 p=0.82). Finally, TP53 VAF did not correlate with RS percentage (p=.393)
Conclusions: In our study, MDS-RS-EB was highly concordant with the presence of TP53 mutation occurring in 80% of SF3B1-wt patients. In TP53 mutant patients, increased ring sideroblast % was an independent covariate associated with a significant survival advantage. As therapy targeting TP53 emerges, the ability to rapidly predict TP53 mutation status based on presence of ring sideroblasts should be a priority.
Komrokji:Agios: Consultancy; celgene: Consultancy; pfizer: Consultancy; DSI: Consultancy; JAZZ: Consultancy; Incyte: Consultancy; Novartis: Speakers Bureau; JAZZ: Speakers Bureau. List:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Sallman:Abbvie: Speakers Bureau; Novartis: Speakers Bureau; Jazz: Research Funding; Incyte: Speakers Bureau; Celyad: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal