Rationale:
Chronic inflammation is a key feature of Myeloproliferative Neoplasm (MPN), inflammation drives symptom burden and disease progression. We have found that monocytes from MPN patients persistently produce Tumor Necrosis Factor-alpha (TNF) following Toll-like receptor stimulation due to dampened Interleukin 10 (IL-10) receptor signaling which usually serves to negatively regulate TNF production (Lai et al, 2019). Chronic inflammation leads to hematopoietic stem cell (HSC) exhaustion, and IL-10 plays an important role in HSC self-renewal (Kang et al, 2007).
JAK2V617Fknock-in HSC do not have a selective advantage over wild-type cells in competitive repopulation assays, this suggests that JAK2V617FHSC gain a selective advantage only under specific circumstances. Here, we investigate whether blockade of IL-10 signaling allows JAK2V617F HSC to gain a selective advantage over wild-type cells. We hypothesize that blocking IL-10 signaling impairs wild-type but not JAK2V617Fmutant HSC and thereby allowing JAK2 V617Fmutant cells to expand.
Results:
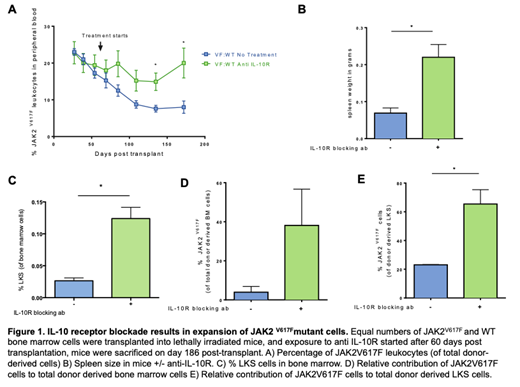
We used competitive transplantation to assess the impact of blocking IL-10 signaling on the selective advantage of JAK2V617Fversus wild-type HSC. We transplanted equal numbers of whole bone marrow cells from wild-type (CD45.1) and JAK2 V617F(CD45.2) mice into lethally irritated recipients (CD45.1/2). At day 60 post transplantation we injected mice intraperitoneally with 0.1mg IL-10R blocking antibody (n=5) or PBS (n=4) weekly. The relative contribution of JAK2V617Fcells to donor derived peripheral blood leukocytes (n=4) decreased over time (Figure 1A) in the PBS group. In contrast, the relative contribution of JAK2V617Fcells increased after 120 days in the IL-10R blocking antibody group (Figure 1A).
Mice were sacrificed at 186 days post-transplant to assess spleen size and quantify hematopoietic stem and progenitor cells. Mice treated with IL-10R blocking antibody had larger spleens than the PBS group (Figure 1B). The LKS (linnegc-kit+Sca-1+), which contains hematopoietic stem and progenitor cells, was expanded in the mice treated with IL-10R blocking antibody (Figure 1C). The relative contribution of JAK2V617Fcells in the LKS compartment (Figure 1D) as well as whole bone marrow (Figure 1E) was higher in the IL-10R blocking antibody group as compared to the untreated group. Bone marrow from these mice were used for secondary transplants to functionally assess HSC fitness, results are forthcoming.
Conclusions:
In vivo blockade of IL-10R signaling increases the competitive ability of JAK2V617Fmutant cells. Spleen size is also augmented with IL-10R blockade, likely a reflection of an increase JAK2V617Fmutant cell burden. This suggests that defects in IL-10 signaling may be contributing to expansion of the mutant cells.
Fleischman:incyte: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal