INTRODUCTION:
The 2016 update of the World Health Organization (WHO) classification criteria for Polycythemia Vera (PV) included a decrease of the hemoglobin threshold. This modification increases exponentially the individuals with potential PV. International groups have proposed diagnostic algorithms for the screening of individuals with potential PV. However, these algorithms are not based on the prevalence of JAK2V617F in this population, and to our concern has not been prospectively validated. Our aim is to develop and validate an algorithm based on previously reported prevalence of JAK2V617F in individuals with potential PV in our environment.
METHODS:
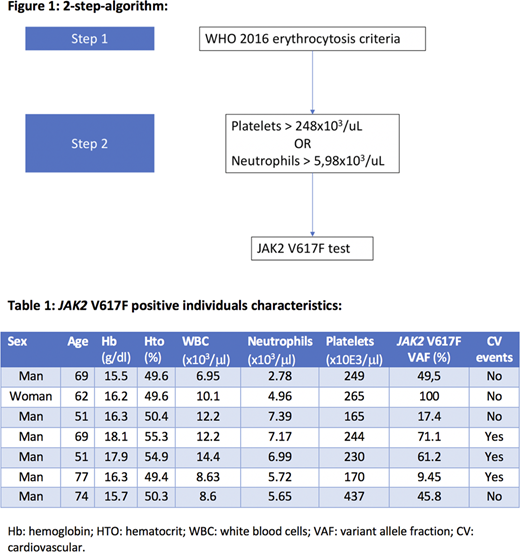
This study was designed in two phases: a first phase for the development of the algorithm and a second phase for the validation of the algorithm. Phase 1: from previous data collected from our group of JAK2 V617F prevalence in individuals with erythrocytosis according to WHO criteria we performed an area under the curve (ROC) analysis with optimal cutoff point in order to maximize sensibility and specificity of the collected variables. The thresholds for platelets and neutrophils were selected for being clinically relevant and with an AUC >0.8. With these two variables and their optimal cutoff point, a sequential 2-step-algorithm was designed (figure 1). Phase 2: for the validation of the algorithm, hemograms from outpatient individuals were selected, and the "2-step-algorithm" was applied. Therefore, if samples met the criteria for step 1 (hemoglobin > 16.5 g/dl for men or 16 g/dl for women or hematocrit >49 % for men or 48 % for women) then, the step 2 was applied (platelets > 248x103/ml or neutrophils>5.98x103/ml). Finally, all samples that passed the 2-step-algorithm, were tested for JAK2 V617F. With the chosen samples a JAK2 V617F qualitative PCR with a sensibility of at least >0.1% was performed. The positive results were validated and quantified in our reference laboratory, and those with an allele burden >1% were considered positive.
RESULTS:
A total of 15298 hemograms were initially selected. Of those, 1595 (10.4%) met the erythrocytosis criterion (step 1). 581 (36%) of these met the step 2 criteria and the JAK2 V617F PCR was performed in 501 samples. A total of 7 positive cases (1.4%) were found, none of which was previously diagnosed of myeloproliferative neoplasm. The median of hemoglobin and hematocrit was 16.3 g/dl and 50.3% respectively. Of the selected samples, 2 of them met the platelet criterion, 4 of them the neutrophils criterion and 1 of them met both. After reviewing clinical records, 43.8% of them were found to have cardiovascular events history history: 1 patient had atherosclerosis of the lower limbs, 1 had acute coronary syndrome and 1 had an ischemic stroke. Characteristics of JAK2V617F individuals are detailed in table 1. Comparing to step 1, the addition of step 2 increases the efficiency of the algorithm 1.75 times. In order to know the prevalence of JAK2 V617F clonal hematopoiesis in our environment, 300 unselected samples were tested. We found 1 positive result. However, after reviewing clinical records, this sample was found to belong to a patient with known myeloproliferative neoplasm. Thus, JAK2 V617F prevalence in our environment was considered <1/300.
CONCLUSION: This "2-step-algorithm" is a reproducible and validated tool for the screening of JAK2 V617F myeloproliferative neoplasms.
Piris-Villaespesa:Novartis: Honoraria, Other: Advisory Boards. Martinez-Lopez:Incyte: Honoraria, Other: Advisory boards; Novartis: Honoraria, Other: Advisory boards; BMS: Honoraria, Other: Advisory boards; VIVIA Biotech: Honoraria; F. Hoffmann-La Roche Ltd: Honoraria; Amgen: Honoraria, Other: Non-Financial Support ; Janssen: Honoraria, Other: Advisory boards and Non-Financial Support ; Celgene: Honoraria, Other: Advisory boards and Non-Financial Support .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal