Background.Transfusion-associated iron overload may increase the risk of infections both increasing bacterial or fungal growth and leading to the production of free iron that impairs immune system response. Anemia and transfusion dependency (TD) represent well known prognostic factors of survival in patients with myelofibrosis (MF). Compared to myelodysplastic syndrome (MDS), the role of iron overload on infection in MF has been scarcely explored.
Methods.We identified consecutive adult patients diagnosed at our Centre with primary or secondary MF, between 1998 and 2018. Patients were stratified in 2 groups according to transfusion dependence defined as having received >2 unit of red blood cells (RBC) over 3 months. The total number of RBC units infused was also collected. All infections were recorded and graded according to Common Terminology Criteria for Adverse Events (CTCAE).
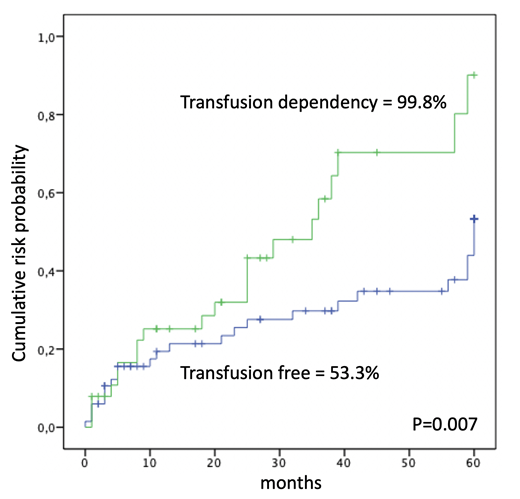
Results.A total of 106 patients, median age 72 years (range 44-89) were retrospectively evaluated. A diagnosis of primary MF was performed in 75 of cases (71%), post Essential Thrombocythemia myelofibrosis (PET-MF) in 27 (25%) and post Polycythemia Vera myelofibrosis (PPV-MF) in 4 (4%). Splenomegaly was present in 83 patients (78%) and 30 (28%) reported constitutional symptoms.According to the IPSS, 43 patients (40 %) were classified at low or intermediate-1 risk, and 63 (60 %) at intermediate-2 or high risk. Molecular analysis was performed in 76 patients (72%): 54 of 76 patients (71%) were positive for the JAK2V617F mutation, 10 (13%) for CALR mutation, 4 (5%) for MPL, and 8 (11%) were negative for all tested mutations. Over time, 56 patients (53%) received hydroxyurea or other cytoreductive treatment, and 23 (22%) ruxolitinib. Overall, 39 patients (37%) were transfusion dependent with a median of 14 RBC units received during follow-up (range 4-199). The median serum ferritin level in the TD cohort was 840 ng/mL (range 130-12.129). Median observation time was 36 months (range 3-203). At last follow-up, 48 patients (45%) presented one or more infectious episodes. Total infectious events were 69 and 13 of them (19%) were defined as severe (grade 3-4 CTCAE). Anatomical site of infections was the respiratory tract in 28 (41%) of cases, gastro-intestinal in 22 (32%), gynecological-urological in 8 (11%), sepsis in 2 (3%), unspecified in the remaining cases. When detected, the etiological agents were bacterial in 8 (12%), viral in 5 (7%) and fungal in 4 (6%). The 60-month cumulative incidence of infection from diagnosis of MF was 64.1±6.5%. TD patients showed a higher incidence of infection (99±8.8% vs 53.3±8%, p=0.007) (Figure 1). In multivariate analysis no association was found between infection incidence and primary vs secondary MF, splenomegaly, age >65 years, ruxolitinib treatment; only transfusion dependencemaintained a significant association (p=0.019; HR=2.13; 95% C.I.=1.13-4.01). Among the 39 TD patients, the transfusion burden was significantly higher in those with infectious complication (median 24 RBC units vs 15 RBC units; p=0.012). The 60-month overall survival was 40±5.9%. Lower IPSS-risk (p<0.0001) and ruxolitinib treatment (p=0.027) were significantly associated with higher survival.
Conclusions.This retrospective monocentric real-life study showed an increased risk of infections in MF patients with higher transfusion burden. The role of iron chelation in improving infection free survival in patients with MF and iron overload should be further investigated.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal