Background
High-grade B-cell lymphoma (HGBL) with MYC and BCL2 and/or BCL6 rearrangements (double- and triple-hit lymphoma), as well as diffuse large B-cell lymphoma (DLBCL), NOS with increased expression of MYC and BCL2 protein (double-expressor lymphoma) are associated with a poor prognosis after front-line treatment with standard immunochemotherapy. As such, therapies targeting MYC and BCL2 alterations are urgently needed. Currently, there are no approved therapies that target MYC. Fimepinostat is an investigational small molecule dual inhibitor of phosphotidyl-inositol-3-kinases (PI3Ks) and Class I and II histone deacetylases (HDACs). Both of fimepinostat's dual mechanisms of action lead to decreased MYC protein: PI3K inhibition leads to enhanced ubiquitin-mediated MYC protein degradation, and HDAC inhibition leads to repression of MYC gene expression. PI3K and HDAC were also inhibited by fimepinostat in peripheral blood mononuclear cells collected from patients (pts) receiving fimepinostat therapy. When dosed in combination with venetoclax in non-clinical studies, fimepinostat demonstrated striking synergistic anti-tumor effects in vitro and in vivo with nearly 100% tumor growth inhibition in a double-hit lymphoma mouse xenograft model (Landsburg 2018a). In clinical studies, fimepinostat +/- rituximab was well tolerated with a favorable safety profile in pts with R/R lymphoma, and resulted in robust and durable objective response rates (ORR) in pts with R/R MYC-altered DLBCL with an ORR of 23% and a median duration of response (DOR) of 13.6 months (Landsburg 2018b).
Study Design and Methods
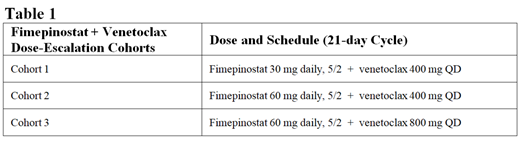
CUDC-907-101 is a Phase 1/2, multi-center, dose-finding study that was recently amended to evaluate fimepinostat in combination with other anti-cancer therapies, including venetoclax. Cohorts of patients will receive increasing dose levels of fimepinostat administered on a 5-days-on-2-days-off (5/2) schedule in combination with venetoclax in 21-day cycles (Table 1). The primary objectives are to determine the maximum tolerated dose, PK, safety and tolerability, and to assess preliminary efficacy, as measured by the ORR and DOR. Eligible pts must have a histologically-confirmed diagnosis of DLBCL or HGBL with or without MYC and/or BCL2 alterations, which is refractory to, or relapsed after, ≥1 prior lines of therapy. Patients must also have an ECOG performance status of 0 or 1, a life expectancy of ≥3 months, measurable disease per Lugano criteria, and have archived or fresh tumor tissue available. Approximately 12 pts in the Ph 1 dose escalation (3+3 design) and 30 pts in the Ph 2 expansion will be enrolled to receive fimepinostat + venetoclax treatment. Patients will be treated until progression or unacceptable toxicity. The Ph 2 expansion will be an estimation study for detecting an efficacy signal. Patients who receive ≥1 dose and have ≥1 post-baseline response evaluation will be included in the efficacy analysis set. Investigator-assessed ORR based on Lugano criteria will be summarized as the proportion of pts who achieve a best response of CR or PR for each combination, and the corresponding two-sided 95% confidence interval (CI, Clopper-Pearson) will be calculated. DOR will be summarized for pts who achieve response using the Kaplan-Meier (KM) product-limit method. The median DOR along with the two-sided 95% CI using the Brookmeyer and Crowley method will be calculated. PFS and OS will be estimated in pts using the KM product-limit method, along with the median and two-sided 95% CI.
The first patient in this study was treated in July 2019, and enrollment is on-going. This new study represents the first clinical trial of the novel-novel combination of fimepinostat with venetoclax in pts with non-Hodgkin lymphoma harboring alterations of both MYC and BCL2. Clinical trial: NCT01742988.
References
a. Landsburg, D. J. et al., Durable Responses Achieved in Patients with MYC-altered Relapsed/Refractory Diffuse Large B-cell Lymphoma Treated with Fimepinostat (CUDC-907): Combined Results from a Phase 1 and Phase 2 Study. Poster presented at: Society of Hematologic Oncology annual meeting. September 12-15, 2018.
b. Landsburg, D. J. et al., (2018). A Pooled Analysis of Relapsed/Refractory Diffuse Large B-Cell Lymphoma Patients Treated with the Dual PI3K and HDAC Inhibitor Fimepinostat (CUDC-907), Including Patients with MYC-Altered Disease. Blood,132(Suppl 1), 4184.
Younes:Epizyme: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Janssen: Honoraria, Research Funding; AstraZeneca: Research Funding; Genentech: Research Funding; Biopath: Consultancy; Xynomics: Consultancy; Syndax: Research Funding; Curis: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Abbvie: Honoraria; Celgene: Consultancy, Honoraria; HCM: Consultancy; BMS: Research Funding; Pharmacyclics: Research Funding; Takeda: Honoraria. Batlevi:Juno Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cohen:Takeda Pharmaceuticals North America, Inc.: Research Funding; Genentech, Inc.: Consultancy, Research Funding; Bristol-Meyers Squibb Company: Research Funding; Seattle Genetics, Inc.: Consultancy, Research Funding; Gilead/Kite: Consultancy; LAM Therapeutics: Research Funding; UNUM: Research Funding; Hutchison: Research Funding; Astra Zeneca: Research Funding; Lymphoma Research Foundation: Research Funding; Janssen Pharmaceuticals: Consultancy; ASH: Research Funding. de Vos:Verastem: Consultancy; Portola Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy. Landsburg:Triphase: Research Funding; Seattle Genetics: Speakers Bureau; Takeda: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Curis, INC: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Curis, INC: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Speakers Bureau; Triphase: Research Funding; Takeda: Research Funding. Patel:Sunesis: Consultancy; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; Pharmacyclics/Janssen: Consultancy, Speakers Bureau. Phillips:Celgene: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Pharmacyclics: Consultancy, Research Funding; Genentech: Consultancy; Incyte: Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy; Seattle Genetics: Consultancy; Bayer: Consultancy. Smith:Portola Pharmaceuticals: Research Funding. Westin:Novartis: Other: Advisory Board, Research Funding; 47 Inc: Research Funding; MorphoSys: Other: Advisory Board; Kite: Other: Advisory Board, Research Funding; Genentech: Other: Advisory Board, Research Funding; Curis: Other: Advisory Board, Research Funding; Juno: Other: Advisory Board; Unum: Research Funding; Celgene: Other: Advisory Board, Research Funding; Janssen: Other: Advisory Board, Research Funding. Ma:Curis, Inc.: Employment. Grayson:Curis, Inc.: Employment. von Roemeling:Curis, Inc.: Employment. Barta:Takeda: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Mundipharma: Honoraria; Seattle Genetics: Honoraria, Research Funding; Bayer: Consultancy, Research Funding; Mundipharma: Honoraria; Merck: Research Funding; Celgene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal