Introduction:
Peripheral T-cell lymphomas (PTCL) represent a heterogeneous group of aggressive non-Hodgkin lymphomas, with suboptimal outcomes with conventional chemotherapy. Autologous hematopoietic stem cell transplant (AHCT) is a therapeutic strategy for patients in first remission. However, progression-free survival (PFS) after AHCT is only 36-45% (d'Amore et al JCO 2012, Reimer et al JCO 2009), signifying an unmet therapeutic need for improving outcomes post-transplant. Maintenance therapy after AHCT may improve PFS. Romidepsin is a histone deacetylase (HDAC) inhibitor that is FDA approved for the treatment of relapsed/refractory T-cell lymphoma, and is a potential option for maintenance therapy. We present the results of the first multicenter study to evaluate the PFS of patients receiving maintenance therapy after upfront AHCT in PTCL patients.
Methods:
This was a phase 2, open-label, multicenter, investigator-initiated study in adult patients with PTCL (Table 1). 25 patients transplanted in first complete response or first partial response (CR1 or PR1) (Cohort 1) were evaluable for the primary endpoint of 2-year PFS. We enrolled another cohort (n=8) with high-risk histologies in CR/PR1 (n= 1), or transplanted in CR/PR2 or later (n=7) (Cohort 2). Patients underwent AHCT with carmustine, etoposide, cytarabine and melphalan (BEAM) conditioning. Maintenance romidepsin 14 mg/m2 was initiated between days 42-80 post AHCT, and administered every other week through 6 months, every 3 weeks through 1 year and every 4 weeks from 1 year through 2 years post AHCT. The Kaplan-Meier method was used to estimate PFS. Desired 2-year PFS was 70%.
Results:
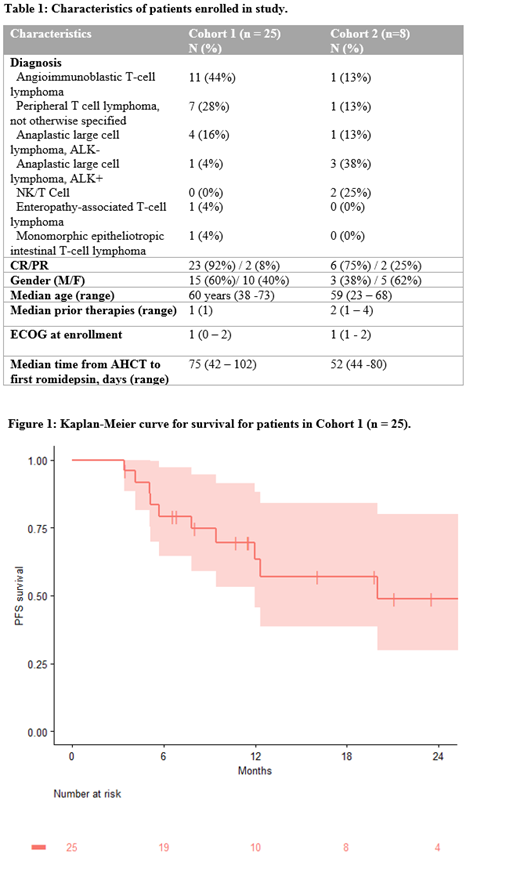
Table 1 lists patient and disease characteristics. In Cohort 1, median follow up was 13.8 months (3.5 - 54.1 mon). Estimated 2-year PFS was 49% (30 - 80, 95% CI) (Figure 1). Median PFS was 20.0 months (12.0- NA, 95% CI). In Cohort 2, median follow up was 23.2 months (range, 9.1 - 35.7 months). Median PFS was 13.9 months (5.6 - NA, 95% CI). Estimated 2-year PFS was 47% (21 - 100, 95% CI). Angioimmunoblastic T-cell lymphoma (AITL) patients represented the largest subgroup within the study. 2-year PFS of these patients in Cohort 1 was 44% (20-96, 95% CI).
In Cohort 1, 16 patients are off therapy (9 for disease progression, 2 for toxicity, 2 for patient choice and 3 completed therapy). Across cohorts, 5 patients required dose reduction. 6 patients experienced ≥ grade 3 toxicity (neutropenia=4, anemia=2, thrombocytopenia=2 and lymphopenia=2). 8 serious adverse events (SAEs) occurred in 6 patients after romidepsin treatment (epistaxis, fever, febrile neutropenia, hypotension, fatigue, myalgia, generalized muscle weakness, dyspnea, and CMV retinitis). Grade 2 toxicities included dysgeusia (5), neutropenia (3), anorexia (2), atrial fibrillation (1), hematuria (1), nausea (1), and fatigue (1). Grade 1 toxicities included dysgeusia (7), fatigue (4), nausea (4), anorexia (2), constipation (2), diarrhea (1), neutropenia (1), thrombocytopenia (1), and vomiting (1).
Conclusions:
Maintenance romidepsin was overall well-tolerated without significant additional grade 3-4 toxicity. At first assessment, the estimated median 2-year PFS in Cohort 1 of 49% does not indicate PFS improvement with romidepsin maintenance. Enrollment is complete and 9 patients in Cohort 1 are still on treatment. Final PFS will be updated.
Khan:ASCO/Conquer Cancer Foundation sponsored by Gilead Sciences: Research Funding; Back Bay Life Science Advisors: Honoraria. Shustov:Seattle Genetics, Inc.: Research Funding. Shadman:Sound Biologics: Consultancy; Sunesis: Research Funding; ADC Therapeutics: Consultancy; BeiGene: Research Funding; Pharmacyclics: Consultancy, Research Funding; Verastem: Consultancy; Celgene: Research Funding; TG Therapeutic: Research Funding; Gilead: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Acerta Pharma: Research Funding; Astra Zeneca: Consultancy; Atara Biotherapeutics: Consultancy; Mustang Bio: Research Funding. Cassaday:Kite/Gilead: Research Funding; Amgen: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Other: Spouse's disclosure: employment, stock and other ownership interests; Incyte: Research Funding. Ruan:AstraZeneca: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie company: Research Funding; Juno: Consultancy; Kite: Consultancy. Moskowitz:Takeda Pharmaceuticals: Consultancy; Merck: Research Funding; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy; Incyte: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Erytech Pharma: Consultancy; Incyte: Research Funding; ADC Therapeutics: Consultancy; Merck: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Incyte: Research Funding; Takeda Pharmaceuticals: Consultancy; ADC Therapeutics: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Incyte: Research Funding; ADC Therapeutics: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; ADC Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Cell Medica: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Erytech Pharma: Consultancy; Erytech Pharma: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Incyte: Research Funding; Merck: Research Funding; Merck: Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Merck: Research Funding; Incyte: Research Funding; Incyte: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Merck: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Cell Medica: Consultancy; Merck: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; ADC Therapeutics: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy; Erytech Pharma: Consultancy; Incyte: Research Funding; ADC Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; Takeda Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Takeda Pharmaceuticals: Consultancy; Takeda Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Merck: Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; Takeda Pharmaceuticals: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding; Cell Medica: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Cell Medica: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Takeda Pharmaceuticals: Consultancy; ADC Therapeutics: Consultancy; Takeda Pharmaceuticals: Consultancy; Incyte: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Merck: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Merck: Research Funding; Cell Medica: Consultancy; Merck: Research Funding; Merck: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Erytech Pharma: Consultancy; Merck: Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Merck: Research Funding; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy; Erytech Pharma: Consultancy; Erytech Pharma: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Incyte: Research Funding; Takeda Pharmaceuticals: Consultancy; Erytech Pharma: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Incyte: Research Funding; Incyte: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Cell Medica: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Incyte: Research Funding; Takeda Pharmaceuticals: Consultancy; Takeda Pharmaceuticals: Consultancy; Incyte: Research Funding; Takeda Pharmaceuticals: Consultancy; ADC Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Erytech Pharma: Consultancy; Cell Medica: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Cell Medica: Consultancy; Erytech Pharma: Consultancy; Erytech Pharma: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding. Zelenetz:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees. Noy:Medscape: Honoraria; Janssen: Consultancy; Prime Oncology: Honoraria; NIH: Research Funding; Pharamcyclics: Research Funding; Raphael Pharma: Research Funding. Straus:Hope Funds for Cancer Research: Membership on an entity's Board of Directors or advisory committees; Elsevier (PracticeUpdate): Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria. Kumar:Seattle Genetics: Research Funding. Sauter:Celgene: Consultancy; Novartis: Consultancy; Genmab: Consultancy; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy; Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy; GSK: Consultancy. Shah:Amgen: Research Funding; Janssen: Research Funding. Matasar:Genentech, Inc.: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding; Bayer: Consultancy, Honoraria, Other; Roche: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding; Merck: Consultancy, Equity Ownership; Juno Therapeutics: Consultancy; Teva: Consultancy; Rocket Medical: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Other: Travel, accomodation, expenses, Research Funding; Daiichi Sankyo: Consultancy; GlaxoSmithKline: Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Bayer: Other: Travel, accommodation, expenses. Van Besien:Miltenyi Biotec: Research Funding. Giralt:Sanofi: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Takeda: Consultancy. Horwitz:Mundipharma: Consultancy; Astex: Consultancy; Trillium: Research Funding; Kyowa Hakko Kirin: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Portola: Consultancy; Aileron: Research Funding; Miragen: Consultancy; Celgene: Consultancy, Research Funding; Kura: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Portola: Consultancy; ADCT Therapeutics: Research Funding; Innate Pharma: Consultancy; Affimed: Consultancy; Aileron: Research Funding; Seattle Genetics: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Infinity/Verastem: Consultancy, Research Funding; ADCT Therapeutics: Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astex: Consultancy; Innate Pharma: Consultancy; Kyowa Hakko Kirin: Consultancy; Seattle Genetics: Consultancy, Research Funding; Mundipharma: Consultancy; Affimed: Consultancy; Forty-Seven: Research Funding; Portola: Consultancy; Trillium: Research Funding; Affimed: Consultancy; Miragen: Consultancy; Astex: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Miragen: Consultancy; Astex: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Mundipharma: Consultancy; Celgene: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Kura: Consultancy; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forty-Seven: Research Funding; Portola: Consultancy; Mundipharma: Consultancy; Aileron: Research Funding; Kura: Consultancy; Celgene: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Kura: Consultancy; Innate Pharma: Consultancy; Aileron: Research Funding; Kyowa Hakko Kirin: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Miragen: Consultancy; Affimed: Consultancy; ADCT Therapeutics: Research Funding; Trillium: Research Funding; Kyowa Hakko Kirin: Consultancy; Forty-Seven: Research Funding; Forty-Seven: Research Funding; Trillium: Research Funding; Innate Pharma: Consultancy; ADCT Therapeutics: Research Funding.
Romidepsin has been FDA approved for the treatment of relapsed/refractory cutaneous T-cell lymphoma and has accelerated approval for treatment of relapsed/refractory peripheral T cell lymphoma. We are studying its use as maintenance therapy after autologous stem cell transplant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal