Background
The time to treatment initiation is determined by tumour burden in patients with follicular lymphoma (FL). The Groupe d'Etude des Lymphomes Folliculaires ('GELF') criteria, defined in the pre-rituximab era, are commonly used to assess tumour burden.2
Patients must meet ≥1 of the following criteria to be considered "high" tumour burden according to GELF: any tumour mass >7 cm; ≥ 3 nodal sites (each >3 cm); B symptoms; splenomegaly; compression syndrome; pleural/peritoneal effusion; leukemic phase or cytopenias.
Low tumour burden FL is often excluded from clinical trials, based on data from initial retrospective studies and later randomised trials, demonstrating no survival advantage with chemotherapy compared with observation alone. 1-3 Conversely, it is recommended those with high burden disease receive immediate therapy. The use of GELF in therapeutic decision-making outside of clinical trials is not well described.
Methods
Cases of newly diagnosed Grade 1-3a FL were retrospectively identified from the Australian Lymphoma and Related Diseases Registry (LaRDR), and 2 additional institutional databases from 2002-2019. Additional data was obtained from electronic hospital records.
The primary aim of the study was to determine the utilisation of GELF criteria in guiding therapeutic decisions in FL. The secondary aims were to document frequency of GELF according to stage and treatment and to determine the impact of the number of GELF criteria on PFS. Survival analysis was calculated according to the Kaplan-Meier method.
Results
385 cases were identified. Patient characteristics are in table 1. The median follow-up was 2 years (range 0.1-18) with 2-year PFS and OS of 89% (95% CI 85-92%) and 96% (95% CI 92-98%) respectively. 94 (24%) patients underwent a 'watch and wait' approach (W&W), 54 (14%) received radiotherapy alone and 237 (62%) received chemotherapy +/- radiotherapy. 118 (31%) patients had stage I/II disease at diagnosis, of these 36 (30%) underwent a W&W approach, 41 (35%) had radiotherapy alone and 41 (35%) received chemotherapy +/- radiotherapy (with only 3 patients in the latter group being enrolled on clinical trial). 260 (69%) had advanced stage disease, of these 56 (22%) underwent a W&W approach, 13 (5%) had radiotherapy alone and 191(73%) received chemotherapy +/- radiotherapy. In the W&W group, 23% of patients had ≥1 GELF criteria, of these, 38% had limited stage disease. Of the patients who received chemotherapy +/-radiotherapy and radiation therapy, 36% and 57% respectively had no GELF criteria identified with 25% and 80% having limited stage disease respectively (table 2)
Where available the reasons for commencing chemotherapy +/- radiotherapy in GELF negative patients were examined and included pain associated with lymph nodes, concerns regarding high-grade transformation (secondary to high SUVmax, size of nodal mass or trajectory of growth), cosmesis, nausea and fatigue.
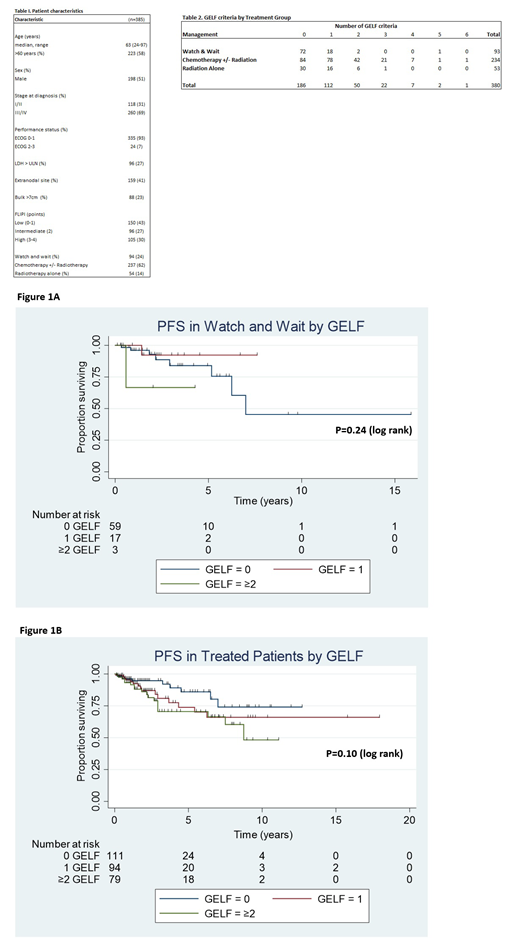
In both the W&W and treated cohorts, the number of GELF criteria did not predict outcome (Figure 1A & B). In a subgroup analysis by treatment modality, patients with no GELF criteria versus those with ≥1 GELF criteria, no statistically significant difference in PFS in the W&W group (PFS: HR 0.69 95% CI 0.14-3.27, P=0.63), the chemotherapy +/- radiotherapy group (PFS: HR 1.66 95% CI 0.79-3.52, P=0.18) and the radiotherapy alone group (PFS: HR 4.53 95% CI 0.51-40.71, P=0.13) was demonstrated.
Conclusion
One fifth of W&W FL patients had ≥ 1 GELF criteria and 36% of those receiving chemotherapy +/-radiotherapy had no GELF criteria at baseline, suggesting clinicians are using other measures to make therapeutic decisions. By restricting eligibility for clinical trials to those with high tumour burden, using GELF, a significant proportion of patients being treated in the 'real world' are not represented. In our cohort of both treated and untreated patients with FL, the presence of ≥1 GELF criteria did not influence prognosis.
References
1. Young RC, et al. The treatment of indolent lymphomas: watchful waiting v aggressive combined modality treatment. Semin Hematol 1988.
2. Brice P, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the GELF. J Clin Oncol 1997.
3. Ardeshna KM, et al; Long-term effect of a W & W policy versus immediate systemic treatment for asymptomatic advanced-stage NHL. Lancet 2003.
Cheah:Roche: Other: Travel expenses; Celgene, Roche, Abbvie: Research Funding; Roche, Janssen, MSD, Gilead, Loxo Oncology, Acerta, BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Keane:Roche: Consultancy, Other: Travel Grant; Celgene: Consultancy; MSD: Consultancy; Gilead: Consultancy; BMS: Research Funding. Johnston:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen, Roche: Membership on an entity's Board of Directors or advisory committees. Dickinson:Merck Sharpe and Dohme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Opat:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Epizyme: Research Funding; CSL: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Research Funding; Mundipharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Amgen: Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy. McQuilten:CSL Biotherapies: Research Funding; Celgene: Research Funding; Gilead Sciences: Research Funding; Takeda Pharmaceuticals: Research Funding; AbbVie: Research Funding; Janssen-Cilag: Research Funding. Wood:Abbvie, Alexion, Amgen, Bristol-Myers Squibb, Celgene, CSL Behring, Gilead, Janssen, Novartis, Roche,, Sanofi, Takeda: Research Funding. Hawkes:Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche/Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Speakers Bureau; Merck KgA: Research Funding; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Research Funding; Takeda: Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Research Funding, Speakers Bureau; Mundi pharma: Research Funding; Astra Zeneca: Research Funding; Merck Sharp & Dohme: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal