Introduction: Serum soluble interleukin-2 receptor (sIL-2R) is recognized as a tumor-related biomarker of malignant lymphomas, including follicular lymphoma (FL). The objective of this study is to assess the prognostic significance of pretreatment serum sIL-2R level for disease-specific overall survival (DSS) in patients with FL.
Methods: We retrospectively analyzed medical records of our 10 individual institutions to identify all patients (pts) ≥ 18 years old with newly diagnosed FL between 01/01/2008 and 12/31/2018. Only pts with FL grade 1-3b, confirmed at pathological review, were included. Pts with histological transformation at diagnosis were excluded. Serum sIL-2R was determined by sandwich enzyme-linked immunosorbent assay. Clinical characteristics, therapies, response, relapse patterns and follow up status were collected and analyzed in the cohort. DSS was defined from the time of initial diagnosis to death due to lymphoma or last follow up. DSS was analyzed according to Kaplan Meier method and differences between subgroups were compared with log-rank test. Multivariate cox regression analysis was used to investigate associations of sIL-2R, FL International Prognostic Index (FLIPI) and progression or relapse within 24 months after diagnosis (POD24) with DSS. Serum levels of sIL-2R was measured before initiation of treatment and the data on the nearest date of the diagnostic biopsy within six months was adopted.
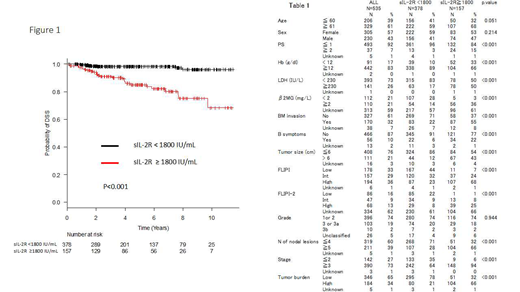
Results: We recorded 565 pts and 535 pts of them with pretreatment serum sIL-2R levels available were included in the analysis with median age 64 years (range: 30-90) and 305 females (57%). Clinical characteristics are summarized in Table 1. The median of the serum sIL-2R level was 896 IU/mL (range: 145 - 23800). We decided an optimal cutoff value of 1800 IU/mL by using receiver operating characteristic (ROC) analysis, which showed it was 1830 IU/mL (AUC : 0.76, 95%CI : 0.67 - 0.84). Various poor prognostic indicators, such as bulky mass, increased LDH, ≥5 nodal lesions, poor performance status (PS), bone marrow invasion, FLIPI and FLIPI-2 high-risk, decreased Hb, advanced disease, high tumor burden, increased β2MG and existence of B symptoms, were strongly associated with high serum sIL-2R level (≥1800 IU/mL), and high sIL-2R level correlated with POD24 (P<0.001, Chi-squared test). The estimated 10-year DSS rate was 87.2% with a median follow up duration of 4.4 years. In pts with POD24 (96 pts, 18%), DSS was significantly worse than those with non-POD24; 64.3% vs 93.0% (P<0.001) and rituximab maintenance did not improve DSS in both groups (P=0.85 and 0.98, respectively). In patients with sIL-2R level of ≥1800 (high sIL-2R, 378 pts, 71%), DSS was significantly worse than those with sIL-2R level of <1800 IU/mL (low sIL-2R, 157 pts, 29%); 68.2% vs 96.0% (P<0.001), respectively (Figure 1). Multivariate analyses employing sex, PS, sIL-2R, FLIPI and POD24 demonstrated that high sIL-2R was an independent prognostic factor for DSS (HR : 2.88, 95% CI : 1.15-7.21, P<0.05). Regardless of POD24 status, pts with high sIL-2R had significantly worse DSS than those with low sIL-2R; 44.6% vs 91.5% in pts with POD24 (P<0.05) and 82.6% vs 96.8% in pts without POD24 (P<0.001), respectively. Among pts treated with rituximab plus anthracycline-containing chemotherapy as the 1st line regimen (222 pts, 41%), pts with high sIL-2R had significantly worse DSS than those with low sIL-2R; 68.1% vs 97.2% (P<0.001). Among pts treated with rituximab-bendamustine as the 1st line regimen (48 pts, 9%), there was no significant difference between high and low sIL-2R populations (P=0.2), however, among pts treated with bendamustine-containing regimen at least once in their life time (180 pts, 34%), there was significant difference between them; 63.1% vs 87.8% (P<0.05). Even in pts achieving complete metabolic response after 1st line treatment (178 pts, 33%), high pretreatment sIL-2R level was a significant factor affecting poor DSS; 75.9% vs 94.3% (P<0.05).
Conclusions: High sIL-2R level ( ≥1800 IU/mL) is a significant and independent adverse prognostic factor for DSS in pts with newly diagnosed FL. This study provides us useful information to predict population with worse DSS before initiation of treatment.
Nozaki:Chugai: Honoraria; Celgene: Honoraria. Kida:Eisai Co., Ltd: Honoraria. Kosugi:Novartis Pharma Co.: Honoraria; Bristol-Myers Squibb Co.: Honoraria; Eisai Co., Ltd: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Kyowa Kirin Co., Ltd: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; ONO PHARMACEUTICAL CO., LTD.: Honoraria; NIPPON SHINYAKU CO.,LTD.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Celgene K.K.: Honoraria; Pfizer Inc.: Honoraria. Shibayama:Astellas, Teijin, MSD, Shionogi, Eisai, Sumitomo Dainippon, Taiho, Nippon Shinyaku: Research Funding; Celgene, Chugai, Eisai, AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Takeda, Novartis, Janssen, Chugai, Eisai, Mundi Pharma, Ono, Otsuka, Kyowa Kirin, Sumitomo Dainippon, AstraZeneca, Avvie, DaiichiSankyo, Fujimoto, Nippon Shinyaku, Sanofi, Bristol-Myers Squibb, Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal