Background: Recent data suggests that older patients with acute myeloid leukemia (AML) have a higher frequency of poor risk TP53 mutations (TP53mut) than previously suspected. Patients with TP53mut have a very poor prognosis, making this AML sub-group an area of high unmet therapeutic need. Although recent clinical trials suggest promising response rates for the BCL-2 inhibitor venetoclax (Ven) in combination with DNA methyltransferase inhibitors (DNMTi) or low-dose cytarabine, survival outcomes remain poor among patients with TP53mut (Strickland et al, EHA 2018).
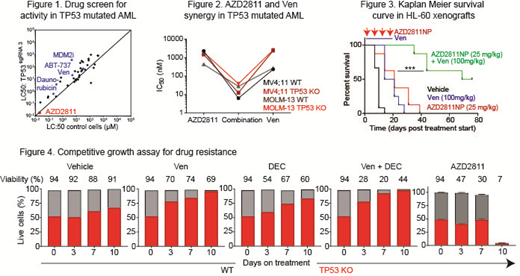
Methods and Results: To identify novel therapies effective against TP53mut AML, isogenic TP53 knockout cells (TP53 KO) were generated by CRISPR/Cas9 in OCI-AML3, MV4;11 and MOLM-13 human AML cell lines. In a drug screen of 50 compounds, we identified AZD2811, an aurora kinase B inhibitor, which potently killed AML cells independently of TP53 genotype (Figure 1). We hypothesised that AZD2811 would synergise with Ven to overcome venetoclax-resistance in TP53-mutant AML. Consistent with this, AZD2811 and venetoclax showed strong synergy (Loewe score <4) in 6 AML cell lines independent of TP53 genotype, and combined treatment displayed highly efficacious activity in both TP53 KO cells and wild type control cells in vitro (Figure 2). Immunoblot of treated cells revealed that AZD2811 depleted MCL-1 expression in the majority of cases, a known cause of venetoclax resistance. AZD2811 was also active in killing Bax/Bak knockout cells, suggesting that the mechanism of AZD2811 may not be limited to the intrinsic apoptosis pathway. Using a competitive growth assay incorporating a fluorescent reporter for TP53 KO cells, we showed the relative resistance of TP53 defective cells to Ven and decitabine (DEC) alone, as well as Ven + DEC, compared to wild type cells. In contrast, TP53 KO cells showed no competitive growth advantage when exposed to an initially sub-lethal dose of AZD2811 (10 nM), compared to TP53 WT cells. Interestingly, continuous exposure to AZD2811 resulted in a catastrophic collapse in cell viability on day 10, unlike comparator anti-leukemic agents (Figure 4). In vivo efficacy for nanoparticle formulated AZD2811 (NP) was observed in SCID mice xenografted with HL-60 cells (known to be TP53 mutant), where combined treatment of AZD2811NP and Ven significantly prolonged animal survival (Figure 3). Remarkably, combined AZD2811NP and Ven treatment was more effective than Ven + azacitidine treatment in vivo. Patient-derived xenograft (PDX) models to verify the activity of AZD2811NP + venetoclax against primary AML cases with TP53 mutations will be presented at the meeting.
Conclusions: We report for the first time that AZD2811NP can overcome venetoclax resistance in TP53-mutant AML in vitro and in vivo. These findings therefore, support the clinical investigation of combined aurora kinase and BCL-2 targeting in the clinic for patients with TP53-mutant AML, who currently lack effective treatment options.
Urosevic:AstraZeneca: Employment. Polanska:AstraZeneca: Employment. Cosaert:AstraZeneca: Employment. Pease:AstraZeneca: Employment, Equity Ownership. Travers:AstraZeneca: Employment. Wei:Pfizer: Honoraria; Janssen: Honoraria; Walter and Eliza Hall Institute: Other: former employee, Patents & Royalties: receives a fraction of its royalty stream related to venetoclax; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Macrogenics: Honoraria; Astellas: former employee, Honoraria; Genentech: Honoraria; Servier: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal