Introduction The BCR-ABL1 gene fusion is the most common molecular aberration in adult ALL and confers an adverse prognosis. The incorporation of ABL1 tyrosine kinase inhibitors (TKIs) into ALL chemotherapy regimens has dramatically improved outcomes for BCR-ABL1+ ALL. Subsequently, reduced-intensity TKI-based regimens have been shown to be as effective and less toxic than intensive TKI-based regimens (Foa et al. Blood 2011; Chalandon et al. Blood 2015). Asciminib (ABL001) is a potent, specific allosteric inhibitor of ABL1 that binds to a site spatially distinct from the catalytic domain. Asciminib was developed for efficacy against BCR-ABL1 mutations conferring resistance to standard TKIs. Preclinical studies demonstrated that the combination of asciminib and TKI leads to sustained disease control in a cell line xenograft model of BCR-ABL1+ CML (Wylie et al. Nature 2017). We also observed that the addition of asciminib to TKI significantly prolongs survival in patient-derived xenograft models of BCR-AB1L+ ALL, even in the context of a pre-existing subclonal T315I mutation (unpublished). An ongoing phase I Novartis study (NCT02081378) has shown safety and efficacy of asciminib alone and in combination with TKIs in relapsed/refractory (R/R) BCR-ABL1+ ALL and CML. We hypothesized that dual ABL blockade with catalytic domain and allosteric inhibitors would be tolerable and effective in older adults with BCR-ABL1+ ALL.
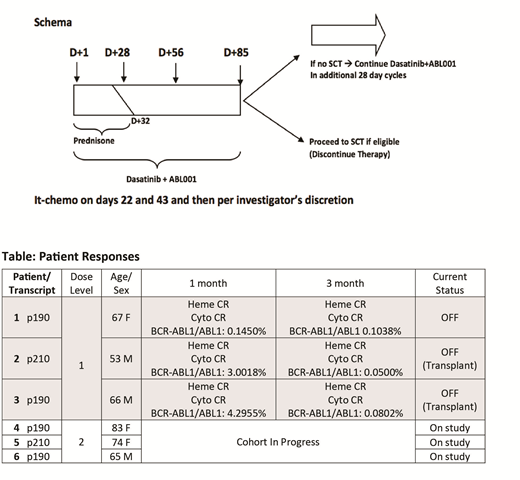
Methods This is an investigator initiated, phase I study (NCT03595917) of asciminib in combination with dasatinib plus prednisone in BCR-ABL1+ ALL. The study uses a 3+3 dose escalation design with an expansion cohort with the primary objective to determine the MTD or RP2D. Secondary objectives include defining the depth of molecular remissions at early time points and durability of responses. Patients (pts) ≥ 50 years (yrs) with newly diagnosed BCR-ABL+ ALL or CML in blast phase with p190 or p210 transcripts are eligible. Younger pts (18-49 yrs) unfit for intensive chemotherapy, or pts ≥ 18 yrs with ALL R/R to intensive chemotherapy are also eligible (excluded if prior dasatinib or asciminib exposure). Pts are treated with dasatinib 140 mg daily and prednisone 60 mg/m2 days 1-24 (max 120 mg daily, tapered days 25-32) following Foa et al with escalating doses of asciminib once daily administered fasting (DL1: 40 mg; DL2: 80 mg; DL3: 160 mg; DL: 320 mg). Pts receive CNS prophylaxis with intra-thecal chemotherapy. Transplant-eligible pts are recommended to be consolidated with allogeneic transplant as medically appropriate at or after day 85; transplant-ineligible pts may remain on therapy if deriving clinical benefit. Correlative science efforts aim to identify biomarkers of response to combined BCR-ABL1 blockade and molecular signatures enriched in minimal residual disease, based on targeted tumor sequencing and serial single-cell RNA sequencing of leukemia and stromal populations from blood and marrow.
Results As of July 31, 2019, 6 adults (3 male/3 female) have enrolled and 3 have completed DL1. All 6 had newly diagnosed, untreated BCR-ABL1+ ALL. The median age at registration was 67 (range 53 - 83) yrs. Three pts treated on DL1 have been evaluated for DLT and discontinued therapy (1 per pt decision after 4 cycles; 2 pts bridged to transplant after 4 cycles). At the time of this report, there has been no induction mortality, no DLTs, and no grade 3 or higher AEs at DL1. At DL1, 1 pt was treated entirely outpatient, the other 2 pts required short hospital stays (4 and 5 days) after treatment initiation. Responses for the 3 pts treated at DL1 are summarized in the table. Pre-treatment tumor genotyping and serial on-treatment, single-cell profiling of tumor and microenvironment fractions from marrow and blood have been performed in all pts to date and have revealed biophysical, transcriptional, and genetic inter-tumor heterogeneity. These data will be used to illuminate disease biology and define predictive biomarkers, pending further maturation of clinical endpoints.
Conclusion Dual ABL1 kinase inhibition with asciminib and dasatinib plus prednisone in newly diagnosed BCR-ABL1+ ALL is feasible in older adults with no increase in toxicity. The last pt at DL2 is completing treatment and being evaluated for DLT before planned escalation to DL3. An expansion cohort of 10 pts at the MTD or RP2D is planned to further characterize safety and clinical activity of the combination.
Stevenson:Celgene: Research Funding. Weinstock:Celgene: Research Funding. DeAngelo:Incyte: Consultancy; Pfizer: Consultancy; Jazz Pharmaceuticals Inc: Consultancy; Novartis: Consultancy, Research Funding; GlycoMimetics: Research Funding; Abbvie: Research Funding; Shire: Consultancy; Celgene: Consultancy; Takeda Pharmaceuticals: Consultancy; Amgen: Consultancy; Blueprint: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal