Chemoresistance is the difference between remission and cure in cancer patients and is particularly evident in acute myeloid leukemia (AML) where complete remissions are common, but few are cured. Genetic analysis in patients points to residual founder clones persisting through therapy and ultimately causing relapse. However, most of these genetic alterations are currently not amenable to targeted therapies. We hypothesized that residual chemoresistant cells must pass through extreme metabolic challenges when under selection from chemotherapy and surrounded by massive cell death. Our goal was to define the distinctive metabolic profile of AML cells as they pass through that time of extreme selection pressure and determine whether metabolic pathway manipulation would reveal an exploitable vulnerability for therapy.
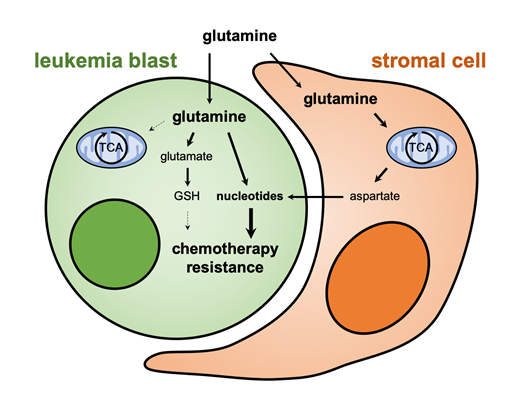
We used in vivo imaging to define the kinetics of leukemic growth, response to chemotherapy and relapse in the mouse. We reasoned that the moment of maximal response immediately following chemotherapy represents a time when the metabolic milieu is maximally hostile and therefore a distinctive selection moment that the cells ultimately causing relapse must pass through. We captured cells at that time and conducted unbiased metabolic profiling, which revealed a metabolite profile that was distinct from AML cells during pre-chemo growth or late post-chemo relapse. Metabolite enrichment analysis revealed hyperactivation of glutamine metabolism during the extreme selection period, with chemoresistant cells showing high levels of glutamine, glutamate and aspartate. Inhibition of glutamine metabolism using the glutamine analog 6-diazo-5-oxo-L-norleucine specifically during the window of maximal selection pressure reduced the number of chemoresistant cells and improved survival in mouse models of AML. Unexpectedly, we found that residual AML cells were not sensitive to inhibition of the enzyme glutaminase, a target currently under clinical investigation for AML. In vivo metabolic tracer analysis instead revealed that chemoresistant AML cells mainly depend on glutamine nitrogen to fuel pyrimidine synthesis. Aspartate, another essential metabolite for pyrimidine synthesis, can be made from glutamine through the Krebs cycle. Interestingly, we detected almost no glutamine carbon entering the Krebs cycle in vivo even though labeled aspartate was found, indicating a different aspartate source. We found that aspartate levels in peripheral blood plasma are very low compared to those in bone marrow plasma, and explored the possibility of a local bone marrow source. Single cell RNA sequencing of bone marrow stromal cells (BMSC) revealed that CXCL12-positive BMSC express high levels of aspartate synthesis genes and transporters, which further increased in the presence of AML cells. In vitro co-cultures showed that BMSC convert glutamine to aspartate, and transfer this to AML cells. Genetic inhibition of aspartate synthesis in BMSC sensitized AML cells to chemotherapy, showing the importance of this metabolic crosstalk.
We propose that induction of a timed metabolic collapse targeting AML cells both directly and indirectly through the bone marrow niche can prevent development of chemoresistance and improve treatment outcomes.
Scadden:Novartis: Other: Sponsored research; Magenta Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Editas Medicine: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Bone Therapeutics: Consultancy; LifeVaultBio: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Consultancy, Equity Ownership; Clear Creek Bio: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Fog Pharma: Consultancy; Red Oak Medicines: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal