Background:
Acute Myeloid Leukemia (AML) is an aggressive hematologic malignancy with known immune dyregulation. In addition to their capacity to rapidly divide, AML cells directly inhibit the activation and proliferation of immune cells in culture. Immunosuppressive features observed in the bone marrow of AML patients include upregulation of Tregs and production of immunosuppressive cytokines (e.g., TGFβ). Irradiating AML cells diminishes their immunosuppressive capacity while maintaining antigen presentation, leading to increased activation of T cells in co-culture. We subsequently identified the immune checkpoint LAG3 as an important mediator of AML-induced immunosuppression and LAG3 modulation as potential treatment strategy.
Methods:
Normal PBMC were isolated from healthy donors. PBMC were co-cultured with non-irradiated and irradiated (40 Gy) human AML cell lines (Kasumi1 (K1), THP1) separately at a 1:2 ratio. On day 3 of co-culture, immunophenotypic characterization of T cells was performed on a flow cytometer using the following surface markers: CD3, CD4, CD8, CD25, CD137, CD154, PD-1, TIM3, TIGIT, and LAG3 and intracellular IFNg and FOXP3. Supernatant from co-culture media were analyzed for cytokine (IL-2, IL-6, IL-10 & TGFβ) secretion by ELISA. CFSE-labeled AML cells were incubated with healthy donor PBMCs in the presence or absence of LAG3, then viability was measured by 7-ADD on flow cytometry.
PBMCs were also isolated from AML patients' peripheral blood and mononuclear cells were isolated from their respective bone marrow samples. Primary AML cultures were established in RPMI complete media with 20% FBS. CFSE-7-ADD killing assay was conducted after incubation of AML cells with autologous PBMCs.
Results:
Healthy donor PBMC co-cultured with irradiated K1 AML cells showed higher intracellular IFNg expression (11.8% ± 3.1 v. 7% ± 3.3; n=7, P=0.012) and higher CD137 expression (9.3% ± 1.21 v. 5.7% ± 3.4; n=7, P<0.001) on CD8+ T cells, and higher CD154 expression on CD4+ cells (44.7% ± 20.3 v. 26.3% ± 14.2; n=5, P=0.002) when compared to the non-irradiated K1-PBMC co-cultures. There were fewer Tregs (CD4+ CD25+ FOXP3+) in the PBMC co-cultured with irradiated K1 cells (1.96% ± 0.37 v. 3.39% ± 0.58; n=4, P=0.03) compared to the non-irradiated K1-PBMC co-cultures. LAG3 expression on CD8+ T cells co-cultured with irradiated K1 was decreased (11.8% ± 2.4 v. 17.5% ± 2.5; n=4, P=0.002) compared to the PBMC co-cultured with non-irradiated K1 cells. No other changes in checkpoint expression on CD8+ T cells were observed. No changes were observed in MHCI or PDL1 expression on non-irradiated K1 AML cells before or after co-culture with PBMC. We observed similar findings with healthy donor PBMC co-cultured with a different AML cell line, THP1; CD137 expression was higher on CD8+ T cells (17.6% v. 6.5%; P=0.02, n=3).
ELISA of the supernatant of culture media showed higher mean OD for secreted TGFβ in the non-irradiated AML co-cultures compared to the irradiated AML co-cultures at 6 hours (2.5 v. 2.0, P=0.03, n=3) and 72 hours (7.9 v. 5.3, P=0.04, n=3). Adding anti-LAG3 antibody (3DS223H; 100 µg/ml) to PBMC co-cultured with non-irradiated AML cells resulted in higher IFNg (16.3% v. 6.6%, P=0.01, n=4) and CD137 expression (6.5% v. 4.1%, p=.007, n=4) on CD8+ cells and fewer Tregs (1.7% v. 3.8%, P=.04, n=4) compared to no antibody added.
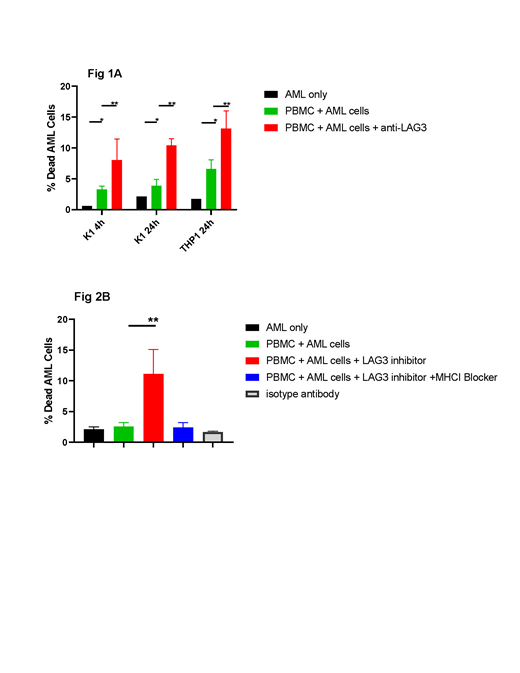
Healthy donor PBMC (n=3) were incubated with CFSE labeled AML cells (K1 and THP1) separately at an effector:target ratio of 5:1. The addition of anti-LAG3 antibody lead to increased killing of both K1 and THP1 AML cells at 4 and 24 hours (Figure 1A). To eliminate the HLA mismatch effect, we incubated PBMC from AML patients with autologous AML cells in the presence or absence of anti-LAG3 (Figure 1B). MHC-I blocking (W6/32, 30 µg/ml) lead to inhibition of cell mediated killing in the presence of anti-LAG3 (Figure 1B).
Conclusion:
In this in vitro model, AML cells showed immunosuppressive features with decreased activation of CD8+ T cells, upregulation of Tregs, increased secretion of TGFβ and higher expression of LAG3 on CD8+ T cells. Antibody blocking of LAG3 mitigated this effect, resulting in increased activation of T cells, fewer T regs and improved MHC-I-mediated killing against AML cells. These results demonstrate that the immunosuppressive effects of AML cells can be modulated through inhibition of LAG-3, suggesting a potential strategy for future combination therapy in AML.
Lin:Jazz Pharmaceuticals: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal