Introduction
Although most individuals with sickle cell disease (SCD) live in sub-Saharan Africa, the history of the disease on this continent remains largely unknown. SCD is characterized by the association of chronic hemolytic anemia with episodes of acute vaso-occlusive events and progressive vascular organ damage. Several pathophysiological pathways in SCD result in the activation of circulating blood cells and the release of microparticles (MPs). In the present study, we investigated cell-derived MPs in patients with SCD living in Africa and analyzed their relationship with clinical complications.
Patients and Methods
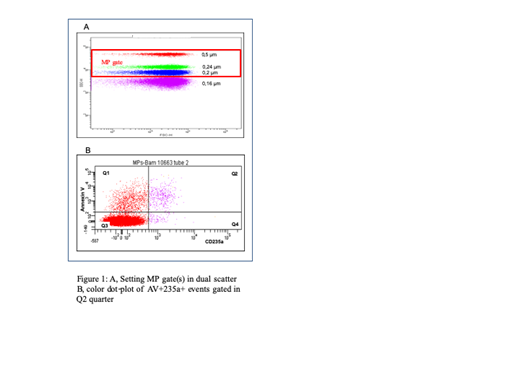
This cross-sectional case-control study is nested in the CADRE cohort (clinical trials.gov identifier NCTO3114137). We included 232 SS adults in two African centers: Bamako (Mali) and Dakar (Senegal). Patients were chosen depending on the absence or the presence of at least one of the following complications: tricuspid regurgitant jet velocity (TRJV) >3 m/s (which may indicate pulmonary hypertension), macroalbuminuria, leg ulcer, priapism, aseptic osteonecrosis, and retinopathy. Overall, 7 groups of 40 SS patients were constituted (20 in each center). Patients were investigated at steady state (i.e.,at least 15 days after a vaso-occlusive crisis, 8 days after fever or infectious disease, and 3 months after a transfusion). MPs were isolated in the African centers immediately after blood sampling by successive centrifugations at increasing speed: 2,500g x2 and 21,000g x2. MPs pellets were stored at -80 °C. The cellular origin of the MPs, erythrocyte, reticulocyte, endothelial, platelet, and leucocyte, was determined using antibodies directed against CD235a, CD71, CD106, CD41, and CD45, respectively, at the National Institute of Blood TransfusioninParis. To maintain the background at an acceptable level, events of 0.16 µm size were excluded (Fig 1A). Only MPs positive for Annexin V and the cell-type-specific labelling were retained. Potential associations between cell-derived MPs, hematological parameters, and vascular complications were assessed using logistic regression with adjustment for age, sex and country.
Results
The MP pellets of 106 SS patients from Bamako and 126 from Dakar were analyzed in Paris. In these patients, at a mean age of 29 years (+/- 11), high TRJV was present in 64, microalbuminuria in 84, leg ulcers in 33, priapism in 43, aseptic osteonecrosis in 45, and retinopathy in 31 patients whereas 28 patients had no complication at the time of sampling. As a typical result, Fig 1B shows erythrocyte-derived MPs labelled by Annexin V and CD235a (quarter Q2). The MPs distribution was as follows: erythroid 52% [reticulocytes (CD235a+CD71+) 14%, erythrocytes (CD235a+ CD71-) 38%], leukocyte 18%, platelet 21%, and endothelial 9%. Neither erythrocyte- nor reticulocyte-derived MPs significantly correlated with hemolysis markers (LDH, unconjugated bilirubin or reticulocytes) or hemoglobin levels. Erythrocyte- and reticulocyte-derived MPs were significantly lower in patients with retinopathy (OR=0.48, p=0.003 and OR=0.68, p=0.005, respectively). Reticulocyte-derived MPs were negatively associated with a history of priapism (OR=0.76, p=0.020) and positively associated with a history of leg ulcers (OR=1.23, p=0.040). No correlation was found between MPs of other cellular origin and chronic complications, except for a negative association between endothelial-derived MPs and priapism (OR=0.76, p=0.036).
Conclusion
In our African patients with SCD, erythroid-derived MPs, although recognized cellular products of hemolysis, were not associated with other markers of hemolysis. We hypothesize that erythroid MPs are not only derived from hemolysis but also probably from the sickling process in this population. They were negatively associated with retinopathy and priapism and positively associated with leg ulcers, but not with the other complications classically associated with the hyperhemolytic sub-phenotype. The search for pertinent biomarkers of SCD complications in Africa is an essential challenge. To our best knowledge, this report is the first illustrating the feasibility of high-technology experiments in an African context.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal