Venous thromboembolism (VTE) is a known complication in children with leukemia, with prevalence in acute lymphoblastic leukemia (ALL) reported as high as 37% (Mitchell, et al, Cancer, 2003). Indwelling catheters, hyperviscosity, mediastinal masses, and medications such as asparaginase, can all contribute to increased VTE risk. In addition to national interest in preventing VTE in hospitalized children, decreasing risk of VTE in children with cancer is of great importance to pediatric hematologist/oncologists. The International Society of Hemostasis and Thrombosis released a guidance statement (Tullius, et al, JTH, 2017) that suggested considering thromboprophylaxis on a case-by-case basis for children with cancer and multiple VTE risk factors, and suggested discontinuing anticoagulant prophylaxis when predisposing conditions resolve. Children with leukemia certainly may have multiple risk factors and therefore deserve consideration for prophylaxis. The Children's Oncology Group is currently conducting a clinical trial studying thromboprophylaxis during induction for ALL. Given the importance of reducing VTE and the interest in thromboprophylaxis, we sought to assess practitioners' practices regarding thromboprophylaxis in children with leukemia.
We performed a cross sectional, anonymous survey of pediatric members of VENUS (the VTE Network of the Hemostasis and Thrombosis Research Society) and ASH (the American Society of Hematology) using Qualtrics, a secure online survey tool. Responses were excluded if physicians answered no to either of 2 screening questions (related to clinical practice and board certification/eligibility), or if no questions were answered after screening. A gift card incentive was offered anonymously. Institutional IRBs approved this study.
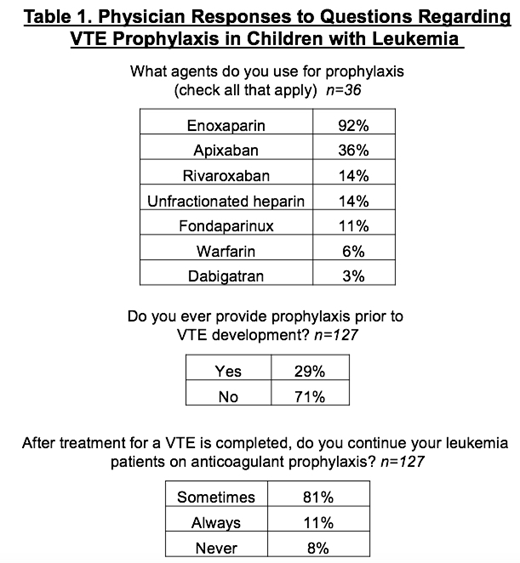
870 surveys were distributed, with a response rate of 17.7%; after exclusions, a total of 142 surveys were included. Demographics were well balanced regarding size of program and years in practice. When asked about which anticoagulant agents may be used for thromboprophylaxis (n=36), 92% of those responding reported using enoxaparin (Table 1). In addition, 36% use Apixaban and 14% use Rivaroxaban.
When asked if they ever provide primary VTE prophylaxis to children with leukemia (n=127), 71% said no, while 29% answered yes (Table 1). When asked in what scenarios primary prophylaxis was used, the most common answer was in leukemia patients with a known inherited thrombophilia (57%). Also common were children with mediastinal masses with vessel compression, and children enrolled on the COG prophylaxis study. Some additional scenarios offered were AYA patients, and those with additional risk factors, such as obesity and immobility. When asked about duration of primary prophylaxis (n=36), the most common times for discontinuing therapy were resolution of mediastinal mass and/or vessel compression (58%), end of all asparaginase therapy (50%), and until the central line is removed (42%). A smaller number of physicians (22%) would consider continuing anticoagulant prophylaxis until the end of all cancer chemotherapy. When questioned about secondary prophylaxis following VTE (n=127), 8% of respondents never provide post-VTE prophylaxis, 11% always give post-VTE prophylaxis, and 81% used post-VTE prophylaxis in certain cases (Table 1.) The most common stopping point for secondary prophylaxis (n=116) was after central line removal (55%), followed by completion of all asparaginase therapy (41%).
Our study is limited by a low response rate. Due to technical issues, we had missing data for many questions and could not obtain free-text answers for some of the prophylaxis questions, limiting full data acquisition. Despite these issues, our results provide some insights into current practices of pediatric hematologist/oncologists. This study shows that many physicians are not utilizing primary anticoagulant prophylaxis in their pediatric leukemia patients, despite the high-risk nature of these patients, possibly trying to balance bleeding risk due to thrombocytopenia. Duration of anticoagulant therapy varies, and it is likely that there are no clear definitions regarding resolution of predisposing factors. Given their unique set of risk factors, and the potential morbidity associated with VTE, further study of VTE prophylaxis is essential for development of pediatric leukemia-specific guidelines.
Cooley:off-label: Other: drug use. Acharya:Bayer Pharmaceuticals, LLC: Research Funding; Novonordisk: Membership on an entity's Board of Directors or advisory committees; BioProducts Laboratory: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees.
We surveyed physicians on which anticoagulants they use in their pediatric leukemia patients and the majority of drugs offered as answer choices are technically off-label.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal