Introduction and Methods
To investigate the mechanisms by which microenvironmental signals induce treatment resistance in chronic lymphocytic leukemia (CLL) we conducted a high-throughput perturbation assay combining pairwise 15 clinically relevant drugs with 18 stimulations mimicking the tumor microenvironment. We combined this with multi-layer omics data using whole exome sequencing, genome-wide DNA-methylation profiles and RNA sequencing to investigate molecular determinants of microenvironmental drug resistance. In parallel we assessed the in-vivo relevance of investigated pathways in 100 CLL infiltrated- and 100 healthy lymph node (LN) biopsies by staining for stimulus specific downstream pathway components using immunohistochemistry (IHC).
Results
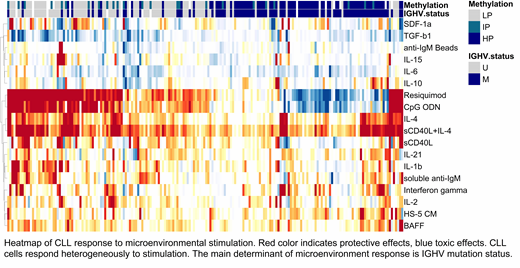
We initially clustered drugs and microenvironmental stimuli separately based on the similarity of their response profiles across all CLL samples. We found drug response profiles to be correlated closely for drugs targeting the same signaling-pathway, e.g. the B-cell receptor inhibitors Ibrutinib (BTK) and PRT062607 (Syk) (Pearson correlation coefficient: r=0.78, 95% CI: 0.72 - 0.83). In contrast, responses to microenvironmental stimuli were less correlated, indicating diverse effects are caused by the tumor microenvironment (e.g. Interleukin 4 (IL-4) vs. Interleukin 2, Pearson correlation coefficient: r=0.17, 95% CI: 0.03 - 0.31).
Twelve of the 18 tested stimulations enhanced survival of CLL cells, whereas two reduced the viability of CLL cells in-vitro (comparisons yielded BH adjusted t-test p-values <0.0001). The strongest pro-survival effects were mediated by IL-4 and Toll-like receptor (TLR) 7/8/9 stimulation, whereas transforming growth factor β1 (TGF-β1) and Interleukin-6 reduced the viability of CLL cells in-vitro.
Ten of the 18 stimulation effects were modulated by genetic alterations in CLL cells, for 6 stimuli two or more response modulating genetic alterations were identified (FDR 10%). IGHV mutation status for instance modulated the effects of TLR 7/8/9 agonists as well as Interleukine-1β, CD40 ligand and TGF-β1. We will present a detailed analysis of how microenvironmental stimuli depend on specific genetic alterations in CLL.
To understand how soluble stimuli interfere with drug response we searched for specific interactions between stimulations and drugs with the following linear model: Viability ~ drug-effect + stimulation-effect + drug-effect:stimulation-effect and tested for significance of the interaction term. This approach allowed us to dissect interactions independently of the individual effects. We discovered different types of drug-effect:stimulation-effect interactions: 1) The microenvironmental stimulus had a strong pro-survival effect on the baseline viability and interfered with the response to drugs (e.g. IL-4:Ibrutinib, p-value< 0.0001). 2) The microenvironmental stimulus had no major effect on baseline viability but significantly interfered with response to drugs (e.g. Interferon-γ:Ibrutinib, p-value: 0.014). 3) The microenvironmental stimulus and the drug exhibited no major individual effect on CLL cell survival, but together significantly increased CLL cell viability (e.g. Interferon-γ:Ralimetinib, p-value: < 0.0001). We will present a detailed analysis of microenvironmental stimuli and their influence on drug response.
In the final step we investigated the biological relevance of selected microenvironmental stimuli in patient LN- and bone marrow biopsies by IHC. CLL infiltrated LN exhibited a higher signaling activity of the IL-4 and TLR pathway than non-neoplastic LN (t-test, p-values <0.0001). A high signaling activity of IL-4 was associated with shorter time to treatment, indicating that CLL progresses faster with strong microenvironmental support.
Conclusion
We present a systematic investigation on the tumor microenvironment in CLL, the underlying molecular determinants as well its interference with drug response which will serve as a scientific resource and starting point for further mechanistical investigations.
Kriegsmann:Celgene: Research Funding; Bristol-Myers Squibb: Research Funding; Sanofi: Research Funding; Morphosys: Research Funding. Müller-Tidow:MSD: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal