Background
LentiGlobin gene therapy contains autologous CD34+ hematopoietic stem cells (HSCs) transduced with the BB305 lentiviral vector (LVV), encoding human β-globin with a T87Q substitution. This substitution confers anti-sickling properties to the gene therapy-derived hemoglobin (HbAT87Q) and allows for its quantification in transduced HSCs. The proof of concept for LentiGlobin gene therapy in patients with transfusion-dependent β-thalassemia (TDT) and sickle cell disease (SCD) was established in the recently completed HGB-205 study (NCT02151526). Herein, we provide the safety and efficacy outcomes and long-term follow-up data for all 7 treated patients, 4 with TDT and 3 with SCD.
Methods
Patients 5−35 years old with TDT (≥ 100 mL/kg of packed red blood cells [pRBCs]/year) or severe SCD (e.g., ≥ 2 acute chest syndromes [ACS] or ≥ 2 vaso-occlusive crises in the preceding year or the year before regular transfusions) were enrolled. CD34+ HSCs were obtained by mobilization and apheresis in patients with TDT or by bone marrow harvest in patients with SCD. Following collection, cells were transduced with the BB305 LVV. Patients underwent busulfan myeloablative conditioning and were infused with transduced cells. Patients were monitored for engraftment, adverse events (AEs), HbAT87Q levels, and other hematologic and clinical parameters. After 2 years in HGB-205, patients transitioned into the long-term follow-up study, LTF-303 (NCT02633943). Summary statistics are shown as median (min-max).
Results
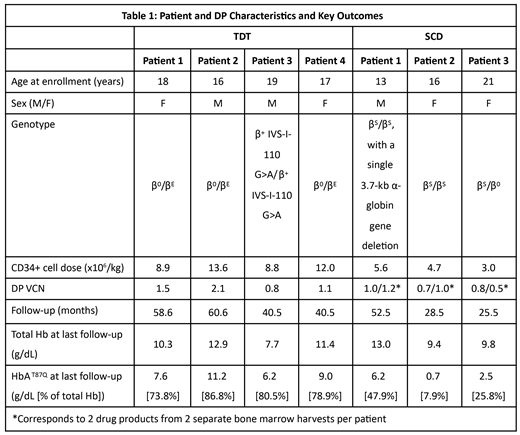
As of June 2019, patients with TDT (n=4) and SCD (n=3) had a median follow-up of 49.6 (40.5-60.6) and 28.5 (25.5-52.5) months, respectively. Table 1 shows patient and drug product characteristics and several key efficacy outcomes. All patients achieved HSC engraftment. LentiGlobin safety profile was consistent with busulfan myeloablative conditioning and, in case of SCD, with the underlying disease state. The most common non-hematologic Grade ≥ 3 AEs post-LentiGlobin gene therapy (≥ 2 patients) for patients with TDT were stomatitis (n=4) and increased aspartate aminotransferase (n=2), and for patients with SCD were ACS (n=2) and vaso-occlusive pain (n=2).
In all 4 patients with TDT, total Hb and HbAT87Q levels remained generally stable up to 5 years post-LentiGlobin infusion. Three of 4 patients achieved transfusion independence (TI; defined as weighted average Hb ≥ 9g/dL without pRBC transfusions for ≥ 12 months), for an ongoing duration of 56.3 (38.2-57.6) months. Weighted average total Hb during TI was 11.4 (10.5-13.0) g/dL. One patient has been off transfusions for 37.5 months and had total Hb of 7.7 g/dL, which was below the ≥ 9 g/dL requirement to meet the protocol definition of TI. At last visit, HbAT87Q levels in these 4 patients ranged from 6.2-11.2 g/dL, which contributed 73.8-86.8% of the total Hb.
The first patient treated with LentiGlobin for SCD experienced one vaso-occlusive pain episode, which developed at 30 months after LentiGlobin gene therapy following a case of acute gastroenteritis with fever and dehydration. The second SCD patient had 2 serious AEs (SAEs) of ACS approximately 6 and 8 months after LentiGlobin gene therapy. The patient resumed chronic pRBC transfusions and hydroxyurea treatment and subsequently experienced 2 SAEs of vaso-occlusive pain; no additional SAEs of vaso-occlusive pain or ACS were reported during the last 16 months of follow-up after LentiGlobin infusion. The third SCD patient had no episodes of vaso-occlusive pain or ACS during 25.5 months of follow-up post-LentiGlobin gene therapy as of the data cut-off. Two patients with SCD who have been off chronic pRBC transfusions, showed improvement in hemolysis markers post-LentiGlobin treatment and stabilization of HbAT87Q expression at approximately 6 months post-LentiGlobin infusion. Total Hb levels for patients with SCD at last visit were 13.0 g/dL (patient 1), 9.4 g/dL (patient 2), and 9.8 g/dL (patient 3), with corresponding HbAT87Q contributions of 47.9%, 7.9%, and 25.8%, respectively.
Summary
With up to 5 years of follow-up, treatment with LentiGlobin gene therapy was well tolerated and resulted in improvement in hematologic parameters and disease-related symptoms. Further results from the completed study will be presented.
Hermine:Celgene: Research Funding; Novartis: Research Funding; AB science: Consultancy, Equity Ownership, Honoraria, Research Funding. Brousse:bluebird bio, Inc: Consultancy; AddMedica: Consultancy. El Nemer:Hemanext: Other: Other. Bartolucci:Novartis: Membership on an entity's Board of Directors or advisory committees; AddMedica: Honoraria, Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; HEMANEXT: Membership on an entity's Board of Directors or advisory committees. Asmal:bluebird bio, Inc: Employment, Equity Ownership. Whitney:bluebird bio, Inc: Employment, Equity Ownership. Gayron:bluebird bio, Inc: Employment, Equity Ownership. Huang:bluebird bio, Inc.: Employment, Equity Ownership. de Montalembert:AddMedica: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; bluebird bio, Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Ribeil:bluebird bio, Inc: Employment, Equity Ownership. Cavazzana:SmartImmune: Other: Founder.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal