INTRODUCTION:
Allogeneic hematopoietic stem cell transplantation (alloSCT) is the only curative strategy for relapsed/refractory T cell lymphoma (T-NHL). In the past ten years, there have been several improvements in conditioning regimens and graft versus host disease prophylaxis (GVHD), which have contributed to lower transplant-related mortality (TRM). Also, selective and low toxicity therapies, might improve response quality in some T-NHL Recently, haploidentical stem cell transplantation (Haplo) with post-transplant cyclophosphamide is a new option for those patients who do not have an HLA-identical sibling or a suitable unrelated donor, but also it has shortened the time for urgent cases.
METHODS:
This study analyzes overall outcomes of 211 consecutive patients diagnosed with T-NHL who received an alloSCT from 1995 to 2018 in GELTAMO/GETH centers. Previous therapies (chemotherapies and autologous stem cell transplantation) and baseline diagnostic parameters were recorded.
RESULTS
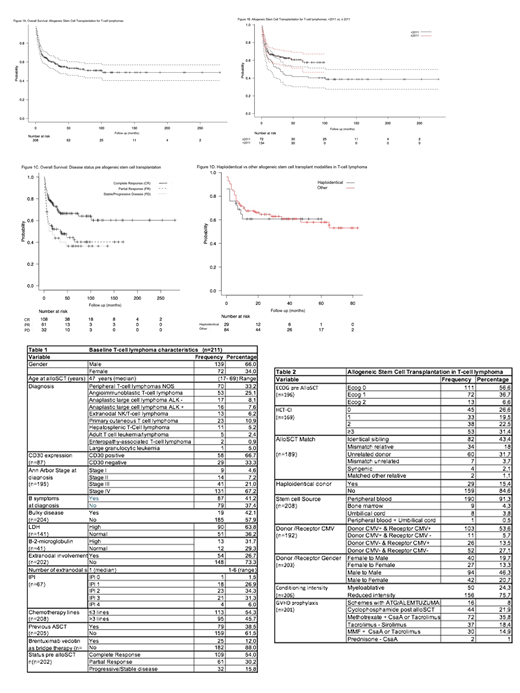
The median age at alloSCT was 47 years (range, 17-69). (see table 1). Forty-nine (23%) had primary extranodal disease. Disease status pre alloSCT was available in 202 patientes: 54% were in complete response (CR), 30% in partial response (PR) and 16% with stable/progressive disease (PD). Since 2013 BV was used as a bridge therapy in ≥ 3rd line in 25 patients with CD30+ tumor expression, it was effective in 20 (CR 68% (n=17), PR 12% (n=3) PD 16% (n=4), not assessed in 1 case). The use of BV was not associated with a better response probability pre alloSCT compared with other regimens used after third line and it did not impact on post alloSCT outcomes. Reduced intensity conditioning (RIC) was the most frequent (76%, n=156). (see table 2) GVHD prophylaxis were Methotrexate + CsaA or Tacrolimus (n=72, 35,8%), sirolimus-tacrolimus (n=37; 18,4%), Cy-post based (n=44, 21,9%; used in Haplo setting n= 29). The median follow-up of all cohort was 22.5 months (range, 0-280). The two year overall survival (OS) and disease free survival (DFS) were 60% (CI95%, 53-67%) and 76.7% (CI95%, 69.3-82.5%) (Figure 1A) We observed a significant improvement in alloSCT outcomes since 2011 (OS <2011 51.4% vs ≥2011 64.8%, p=0,04).(Figure 1B)
Disease status was the only pre alloSCT variable that impacts 2 years - OS: CR 72.8% (CI95%, 63-80.4%), PR 52%(38.7-63.7%), PD 43.8 (26.5-59.8%) (p=0.002) (Figure 1C). Forty-three (21%) cases relapsed after alloSCT.
To analyze the impact of GVHD on OS and DFS we selected landmark time point at day +100 and +1 year after alloSCT for acute (aGVHD) and chronic GVHD (cGVHD) respectively, which allowed us to capture the majority of events that could interfere with the analysis. A landmark analysis (day +100) showed a 2 year OS for grade 3-4 aGVHD was 18% and for 1-2 aGVHD 54,6% (p<0,001). The severity of aGVHD had no impact on DFS. Different grades of cGVHD did not impact OS nor DFS significantly.
Cumulative incidence of acute GVHD at 90 days was 51.6% (CI95%, 43.9-58.2%) being 27% grade 3-4. Chronic GVHD at 6 months was 53.9% (46.1-60.5), 54% of cases were grade 3-4). The 2 years non relapse mortality (NRM) was 30.2% (CI95%, 23.3-36.5%); the main causes contributing to NRM were GVHD (40%) and infections (44%)
Haploidentical (Haplo) alloSCT was introduced in 2012 (29 of 128). With a median follow up of 13 months (range, 0-60) we found that outcomes in terms of 1 year OS (Haplo 60.7% vs. others 67,5%), 1 year DFS (Haplo 74.8% vs. others 83.8%) and 1 year NRM (Haplo 29.7% vs. 26%) are similar to other alloSCT modalities (Figure 1D). Not additional analysis could be estimated due to the low number of population at risk for each category.
CONCLUSION
Overall outcomes of alloSCT for T-NHL have improved over time. Complete response pre alloSCT is the only determinant for OS. Haploidentical alloSCT is not significantly different from other approaches and should be considered as an alternative.
Sierra:Novartis: Honoraria, Research Funding, Speakers Bureau; Astellas: Honoraria; Pfizer: Honoraria; Daiichi-Sankyo: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Roche: Honoraria; Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal