
Background
The best preparative regimen for the growing number of older acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) patients undergoing allogeneic hematopoietic cell transplantation (HCT) from matched related (MRD) or unrelated donors (MUD) remains undefined. A large randomized phase III trial (MC-FludT.14/L study: ClinicalTrials.gov Identifier: NCT00822393) recently demonstrated that myeloablative intravenous (IV) treosulfan (10 g/m² IV on days -4 to -2) in combination with fludarabine (TreoFlu) improves outcome in older and/or comorbid patients with AML in complete remission (CR) or MDS compared with the reference reduced intensity busulfan (0.8 mg/kg IV in 6-hour intervals on days -4 and -3) and fludarabine (30 mg/m² IV on days -6 to -2 in each study arm) regimen. The beneficial effect of the TreoFlu regimen resulted from a significantly reduced non-relapse mortality (NRM) and translated to improved event-free survival (EFS) and overall survival (OS) (Beelen DW et al The Lancet Haematology, 2019). These results raised the question, how this new regimen compares to broadly applied myeloablative regimens, namely busulfan (0.8 mg/kg IV in 6-hour intervals over 4 days) plus cyclophosphamide (120 mg/kg IV over 2 days) (BuCy) or melphalan (140 mg/m² IV over 1 day or 2 days) plus fludarabine (MelFlu) in older AML and MDS patients. To address this question, we performed a comparative analysis of MC-FludT.14/L study patients treated with the TreoFlu regimen and similar patients of the European Blood and Marrow Transplantation Society (EBMT) registry, who underwent HCT from MRD or MUD after the BuCy or MelFlu regimen between 2010 and 2016.
Patients and Methods
Inclusion criteria were essentially the same as for the MC-FludT.14/L-study (patient age 50 to 70 years [yrs], primary or secondary AML in CR or MDS, Karnofsky-index ≥ 60%, MRD or MUD, 1st HCT). The study objectives were to compare OS, relapse incidence (RI), and NRM at 2 yrs after HCT between the TreoFlu regimen and the BuCy or MelFlu regimen. A total of 1493 EBMT registry patients (median age 58 yrs, AML: n=1135 [76%], MDS: n=358 [24%]) were identified for the comparison with the 252 MC-FludT.14/L patients (median age 61 yrs, AML: n=174 [69%], MDS: n=78 [31%]). A 1:1 matching method based on propensity scores (PS) with 14 patient-, donor-, and disease-characteristics was used to reduce confounding due to differences between regimens and was performed separately for AML and MDS patients. With the exception of comparison between the TreoFlu and BuCy regimen in AML patients, a significantly higher proportion of patients in the TreoFlu regimen subsets had a HCT-comorbidity index > 2 compared to patient subsets treated with the BuCy or MelFlu regimen.
Results
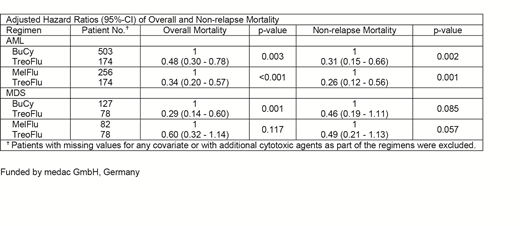
For patients with AML, the 2-yrs OS estimate was significantly higher after the TreoFlu compared with the BuCy regimen (76.4%, 95%-confidence interval [95%-CI]: 66.8% - 85.9% vs 49.2%, 95%-CI: 36.4% - 62.1%, p<0.001) and MelFlu regimen (72.7%, 95%-CI: 63.7% - 80.7% vs 58.7%, 95%-CI: 48.3% - 69.1%, p=0.04). This was clearly related to the significantly lower 2-yrs NRM estimate after the TreoFlu compared with the BuCy regimen (3.9%, 95-CI: 0% - 8.2% vs 23.5%, 95%-CI: 13.1% - 33.9%, p<0.001) and MelFlu regimen (6.4%, 95-CI: 1.8% - 11.0% vs 17.5%, 95%-CI: 9.6% - 25.5%, p<0.02). For patients with MDS, the differences of the 2-yrs OS and NRM estimates between patient subsets were similar, but were only significant for the comparison of the OS estimate between the TreoFlu and BuCy regimen (72.0%, 95%-CI: 54.4% - 89.6% vs 30.5, 95%-CI: 6.1% - 54.9%, p<0.01). The adjusted hazard ratios of 2-yrs overall mortality and NRM between regimens including all eligible patients by multivariate sensitivity analysis based on Cox proportional hazards models are given in the table below. Notably, no differences between the respective 2-yrs RI were detectable when comparing these patient subsets.
Conclusion
In older AML and MDS patients, the new TreoFlu regimen compares favorable to the broadly applied myeloablative BuCy and MelFlu regimens. The substantially lower 2-yrs NRM estimate supports its superior tolerability in this patient population. This large retrospective comparative analysis provides a basis for properly designed randomized trials of the new toxicity reduced myeloablative TreoFlu regimen in comparison with other myeloablative regimens in this target population.
Beelen:Medac GmbH Wedel Germany: Consultancy, Honoraria. Stoelzel:Neovii: Other: Travel funding; Shire: Consultancy, Other: Travel funding; JAZZ Pharmaceuticals: Consultancy. Dreger:MSD: Membership on an entity's Board of Directors or advisory committees, Other: Sponsoring of Symposia; Neovii, Riemser: Research Funding; AbbVie, Gilead, Novartis, Riemser, Roche: Speakers Bureau; AbbVie, AstraZeneca, Gilead, Janssen, Novartis, Riemser, Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal